Why Does Electronegativity Increase From Left To Right
Juapaving
Mar 27, 2025 · 6 min read
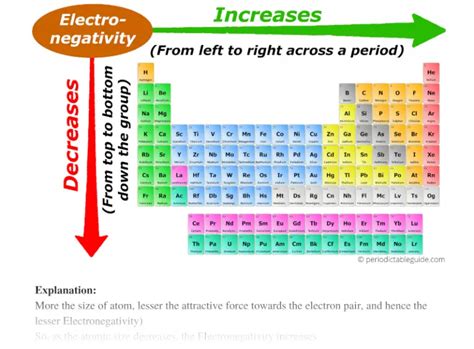
Table of Contents
Why Does Electronegativity Increase From Left to Right Across a Period?
Electronegativity, a fundamental concept in chemistry, describes an atom's ability to attract shared electrons in a chemical bond. Understanding the periodic trends of electronegativity is crucial for predicting the nature of chemical bonds and the properties of molecules. One of the most significant trends is the increase in electronegativity from left to right across a period (row) of the periodic table. But why does this happen? This article delves deep into the atomic structure and the forces at play to explain this crucial periodic trend.
The Role of Effective Nuclear Charge
The primary driver behind the increase in electronegativity across a period is the effective nuclear charge. This refers to the net positive charge experienced by an electron in an atom. It's not simply the total number of protons in the nucleus (atomic number), but rather the positive charge felt by the outermost electrons after considering the shielding effect of inner electrons.
Shielding and Penetration
As we move across a period, the number of protons in the nucleus increases. Simultaneously, electrons are added to the same principal energy level (shell). While these added electrons experience some degree of repulsion from each other (electron-electron repulsion), they are also increasingly drawn towards the larger positive charge of the nucleus. However, the added electrons are not perfectly effective at shielding each other from the nuclear charge. This imperfect shielding is due to the probability distributions of electrons – some orbitals penetrate closer to the nucleus than others, experiencing a stronger pull.
Key takeaway: The increase in nuclear charge outweighs the increase in electron-electron repulsion across a period. The electrons added to the outermost shell don't fully shield each other from the growing positive charge at the center. This results in a progressively stronger pull on the valence electrons.
Atomic Radius and Electronegativity: An Inverse Relationship
Another crucial aspect is the atomic radius. As we move from left to right across a period, the atomic radius generally decreases. This is because the increased effective nuclear charge pulls the electrons closer to the nucleus, resulting in a smaller atom.
This decrease in atomic radius has a direct impact on electronegativity. The closer the valence electrons are to the nucleus, the stronger the attraction between them and the positively charged protons. A smaller atom means a stronger pull on the shared electrons in a bond, hence a higher electronegativity.
In essence: The increase in electronegativity from left to right is a consequence of the decreasing atomic radius, which itself is a result of the increasing effective nuclear charge. The two are inversely related; a smaller atomic radius translates to higher electronegativity.
Electron Configuration and Electronegativity
The electron configuration of atoms plays a significant role in determining their electronegativity. As we traverse a period, electrons are added to the same principal energy level, gradually filling the available orbitals. This filling follows specific rules, most notably the Aufbau principle and Hund's rule.
Filling Orbitals and Nuclear Attraction
When orbitals are partially filled, the added electrons experience less shielding from their peers. However, as we get closer to a completely filled subshell (like a filled p-subshell), the electrons are more effectively shielded. The exceptions to this general trend are usually minimal, and the overall trend of increasing electronegativity across a period remains dominant.
The addition of electrons to the outermost shell doesn’t increase shielding proportionally; the increased attraction to the nucleus overpowers the additional electron-electron repulsion.
The Octet Rule and Stability
The desire of atoms to achieve a stable electron configuration (often an octet, eight electrons in their outermost shell) contributes to their electronegativity. Atoms with nearly filled valence shells have a stronger pull on electrons to achieve this stable configuration, thereby demonstrating higher electronegativity.
Exceptions and Irregularities
While the trend of increasing electronegativity from left to right across a period is generally true, there are some minor exceptions and irregularities. These are often subtle and are usually explained by factors like the electron configuration details, particularly the relative stability of half-filled and fully filled subshells. The subtle differences are generally not large enough to negate the overall trend.
Comparing Electronegativity Across Periods: Specific Examples
Let's examine a specific period, for example, period 2 (Li to Ne), to solidify our understanding:
- Lithium (Li): Has a low electronegativity because it has only one valence electron and a relatively large atomic radius. The single valence electron is easily lost to achieve a stable electron configuration.
- Beryllium (Be): Shows slightly higher electronegativity than Li, but still relatively low, due to its two valence electrons.
- Boron (B): Displays increased electronegativity compared to Be, reflecting the smaller atomic radius and increasing effective nuclear charge.
- Carbon (C), Nitrogen (N), Oxygen (O), Fluorine (F): These elements display a progressive increase in electronegativity as the effective nuclear charge increases and the atomic radius shrinks. The increasing attraction to the nucleus becomes more significant.
- Neon (Ne): A noble gas with a full valence shell, Neon has no tendency to gain or share electrons and thus is assigned a very high electronegativity value; However, it practically has no electronegativity because it doesn't participate in typical covalent bonding.
This pattern of increasing electronegativity across the period 2 demonstrates the overall trend perfectly. Similar patterns can be observed in other periods, although the magnitude of the increase may vary slightly.
Applications and Importance of Understanding this Trend
Understanding the trend of increasing electronegativity across a period is vital for:
- Predicting bond polarity: The difference in electronegativity between two atoms determines the polarity of the bond they form. A large difference leads to polar covalent bonds, while a small difference results in nonpolar covalent bonds.
- Determining molecular geometry: The polarity of bonds influences the overall molecular geometry and properties like dipole moments.
- Understanding chemical reactivity: Electronegativity influences the reactivity of elements and their tendency to form certain types of compounds.
- Interpreting spectroscopic data: Electronegativity differences can affect the vibrational frequencies of bonds observed in infrared spectroscopy.
Conclusion: A Summary of Electronegativity Trends
The increase in electronegativity from left to right across a period is a fundamental periodic trend driven primarily by the increasing effective nuclear charge. As protons are added, the valence electrons experience a stronger pull toward the nucleus, leading to smaller atomic radii and greater attraction for shared electrons in a chemical bond. While minor exceptions and irregularities might be present due to the complexities of electron configuration, the overall trend is clear and robust, playing a crucial role in understanding chemical bonding and molecular properties. This knowledge forms the basis for predicting chemical behavior and interpreting various experimental observations in chemistry. From simple bond polarity estimations to complex molecular behavior analysis, understanding electronegativity trends empowers a deeper and more accurate understanding of the fascinating world of chemistry.
Latest Posts
Latest Posts
-
Lowest Common Multiple Of 6 And 7
Mar 30, 2025
-
Starch Cellulose And Glycogen Are Alike In That They
Mar 30, 2025
-
Does A Circle Have Line Symmetry
Mar 30, 2025
-
What Ocean Is Between North America And Europe
Mar 30, 2025
-
There Is A Repulsive Force Between Two Charged Objects When
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about Why Does Electronegativity Increase From Left To Right . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
