Starch Cellulose And Glycogen Are Alike In That They
Juapaving
Mar 30, 2025 · 6 min read
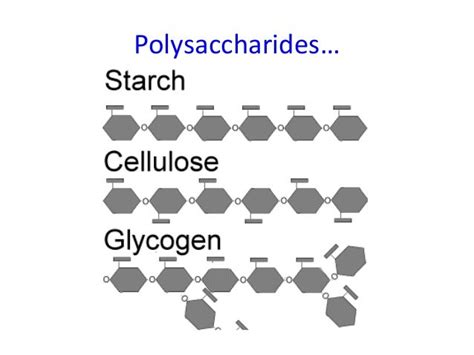
Table of Contents
Starch, Cellulose, and Glycogen: A Comparative Look at Their Similarities and Differences
Starch, cellulose, and glycogen are all polysaccharides, meaning they are complex carbohydrates composed of long chains of simpler sugar units called monosaccharides. While they share this fundamental similarity, their structures and functions differ significantly, leading to their diverse roles in living organisms. This article will delve deep into the similarities and differences between these crucial biological molecules, exploring their structures, functions, and the implications of these variations.
Similarities: The Common Thread of Glucose
The most striking similarity between starch, cellulose, and glycogen lies in their building block: glucose. All three polysaccharides are polymers of glucose, meaning their long chains are composed of repeating glucose units. This fundamental similarity underpins many of their shared properties, such as their role as energy storage or structural components. However, the type of glucose and how these glucose units are linked are where the significant differences emerge.
1. Glucose as the Monomer:
Each glucose molecule is a six-carbon sugar with the chemical formula C₆H₁₂O₆. While the molecular formula is the same, the arrangement of atoms within the molecule can vary, resulting in different isomers like α-glucose and β-glucose. This seemingly subtle difference has profound implications for the properties of the resulting polysaccharides.
2. Energy Storage (Primary Function in Starch and Glycogen):
Both starch and glycogen serve as primary energy storage molecules in living organisms. Starch is the primary energy storage polysaccharide in plants, providing a readily available source of glucose for metabolic processes. Glycogen, on the other hand, is the main energy storage polysaccharide in animals and fungi, fulfilling a similar role. This shared function reflects their ability to be easily broken down into glucose when energy is needed.
3. Hydrophilic Nature:
Due to the presence of numerous hydroxyl (-OH) groups in the glucose units, all three polysaccharides exhibit some degree of hydrophilicity (affinity for water). This property is critical for their solubility in aqueous environments, essential for their metabolic processes and cellular functions.
Differences: Structure Dictates Function
Despite their shared building block, significant differences in the structure of starch, cellulose, and glycogen determine their distinct functions. These differences primarily arise from the type of glycosidic linkages between glucose units and the overall branching patterns of the polymer chains.
1. Glycosidic Linkages: α vs. β
The crucial difference lies in the type of glycosidic linkage between the glucose units. Starch and glycogen are composed of glucose units linked by α-1,4 glycosidic bonds, meaning the bond forms between carbon atom 1 (in the α configuration) of one glucose molecule and carbon atom 4 of another. This creates a relatively compact structure that can be easily broken down by enzymes.
Cellulose, however, is composed of glucose units linked by β-1,4 glycosidic bonds, a key distinction. This seemingly small change in the orientation of the bond drastically alters the properties of the polymer. The β-linkage results in a straight, rigid chain, leading to the formation of strong, insoluble fibers.
2. Branching Patterns: Linear vs. Branched
Starch exists in two main forms: amylose and amylopectin. Amylose is a linear polysaccharide with α-1,4 linkages, while amylopectin is branched, with additional α-1,6 linkages creating branch points approximately every 24-30 glucose units. This branching allows for efficient storage and rapid breakdown of glucose molecules.
Glycogen is even more highly branched than amylopectin, with branches occurring approximately every 8-12 glucose units. This extensive branching allows for more rapid mobilization of glucose when energy is required, which is crucial for the quick energy demands of animals.
Cellulose, in contrast, is a linear polysaccharide without branching. This linear structure, combined with β-linkages, allows cellulose molecules to form strong hydrogen bonds with each other, resulting in the formation of highly stable and insoluble microfibrils. These microfibrils provide the structural rigidity of plant cell walls.
Function and Biological Significance
The structural differences between starch, cellulose, and glycogen directly relate to their distinct roles in living organisms:
1. Starch: Plant Energy Storage
Starch serves as the primary energy storage molecule in plants. Its branched structure (amylopectin) allows for rapid release of glucose molecules when energy is needed, while the linear structure (amylose) provides a more compact storage form. The ease with which enzymes can break down the α-linkages makes starch a readily accessible energy source for plant metabolism.
2. Cellulose: Plant Structural Support
Cellulose forms the main structural component of plant cell walls. The linear structure and β-1,4 linkages result in strong, insoluble fibers that provide structural support and protection for plant cells. The inability of most animals to digest cellulose (lack of cellulase enzymes) makes it an important dietary fiber.
3. Glycogen: Animal Energy Storage
Glycogen serves as the primary energy storage polysaccharide in animals and fungi. Its highly branched structure allows for rapid mobilization of glucose during periods of high energy demand, ensuring a quick supply of energy for muscle contraction, brain function, and other metabolic processes. Glycogen is stored primarily in the liver and muscles.
Enzymes and Digestion
The differing glycosidic linkages also influence how these polysaccharides are digested. Animals possess enzymes (amylases) that efficiently break down the α-1,4 linkages in starch and glycogen, releasing glucose for energy production. However, most animals lack the enzyme cellulase, which is necessary to break down the β-1,4 linkages in cellulose. Therefore, cellulose passes largely undigested through the animal digestive system, acting as dietary fiber. Certain microorganisms, however, possess cellulase, enabling them to digest cellulose.
Applications and Technological Significance
Our understanding of the properties of starch, cellulose, and glycogen has led to numerous technological applications:
- Starch: Widely used in food processing as a thickener, stabilizer, and binder. Also used in the production of biofuels and biodegradable plastics.
- Cellulose: Used in the production of paper, textiles, and various other materials. Increasingly being explored as a sustainable source of biofuels and building materials. Modified cellulose derivatives are used in various industrial applications.
- Glycogen: Used in some food products as a thickener or stabilizer, although its primary importance is in its biological role. Research is ongoing into potential applications of glycogen in drug delivery systems and other biomedical fields.
Conclusion: A Tale of Two Linkages
Starch, cellulose, and glycogen, while all being glucose polymers, demonstrate the remarkable power of subtle structural differences to dictate vastly different biological functions. The distinction between α-1,4 and β-1,4 glycosidic linkages is paramount, shaping their roles as energy storage molecules (starch and glycogen) and a structural component (cellulose). Understanding these nuances allows for harnessing their unique properties in various applications, contributing to advances in food science, materials science, and biotechnology. Further research continues to unlock new potentials and possibilities of these fundamental biological molecules. The seemingly simple glucose molecule, through its diverse linkage arrangements, illustrates nature's elegant design and efficiency in creating a vast array of functional biomolecules.
Latest Posts
Latest Posts
-
Least Common Multiple 12 And 18
Apr 01, 2025
-
What Phase Is The Reverse Of Prophase
Apr 01, 2025
-
Is Tungsten A Metal Or Nonmetal
Apr 01, 2025
-
How Many Seconds Is 24 Hours
Apr 01, 2025
-
What Is The Function Of The Collecting Duct
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Starch Cellulose And Glycogen Are Alike In That They . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
