Where Are The Metals In The Periodic Table Found
Juapaving
Mar 04, 2025 · 7 min read
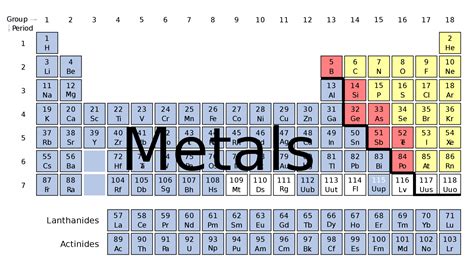
Table of Contents
Where Are the Metals in the Periodic Table Found? A Comprehensive Guide
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic number and recurring chemical properties. A significant portion of these elements are metals, exhibiting characteristic properties like conductivity, malleability, and ductility. But where exactly are these metallic elements located within this organized arrangement? This comprehensive guide will delve into the periodic table's structure, explore the location of various metal types, and discuss the underlying reasons for their placement.
Understanding the Periodic Table's Structure
Before diving into the location of metals, let's briefly review the periodic table's structure. The table is arranged in periods (rows) and groups (columns). Periods represent the principal energy levels of electrons, while groups represent elements with similar outer electron configurations, leading to similar chemical behaviors. This organization isn't arbitrary; it reflects the underlying quantum mechanical principles governing electron arrangements within atoms.
The table is further divided into sections based on the electron configuration and properties of the elements:
-
Main Group Elements (s and p blocks): These elements occupy the left and right sides of the table. The s-block elements are found in groups 1 and 2, while the p-block elements are located in groups 13-18. Both s and p blocks contain a mix of metals, nonmetals, and metalloids (elements exhibiting properties of both metals and nonmetals).
-
Transition Metals (d block): These elements, found in groups 3-12, are characterized by partially filled d orbitals in their atoms or ions. They are primarily metals, known for their variable oxidation states and the formation of colorful compounds.
-
Inner Transition Metals (f block): This block is typically shown separately below the main table. It includes the Lanthanides (rare earth elements) and Actinides, characterized by partially filled f orbitals. These are almost exclusively metallic elements.
Locating Metals in the Periodic Table: A Visual Guide
The vast majority of metals reside on the left and center of the periodic table. Let's break this down by metal category:
1. Alkali Metals (Group 1): A Highly Reactive Family
The alkali metals, including lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr), are found in the first column (Group 1) of the periodic table. They are extremely reactive metals, readily losing one electron to form +1 ions. Their reactivity increases down the group, due to the increasing atomic size and decreasing ionization energy. This high reactivity means they are rarely found in their elemental form in nature.
2. Alkaline Earth Metals (Group 2): Less Reactive, but Still Metallic
The alkaline earth metals, including beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra), occupy the second column (Group 2) of the periodic table. These metals are less reactive than alkali metals, losing two electrons to form +2 ions. Their reactivity also increases down the group, although less dramatically than in Group 1.
3. Transition Metals (Groups 3-12): A Diverse Group with Varying Properties
The transition metals form a significant portion of the periodic table's center, comprising groups 3 through 12. This large group exhibits a wide range of properties. They are generally good conductors of heat and electricity, possess high melting and boiling points, and often form colored compounds. Many transition metals are crucial in industrial applications and biological systems. The variability in their properties stems from the variable oxidation states they can adopt, due to the involvement of d-electrons in chemical bonding.
4. Inner Transition Metals (f-block): The Lanthanides and Actinides
The inner transition metals, consisting of the Lanthanides (rare earth elements) and Actinides, are located separately at the bottom of the periodic table. The Lanthanides are characterized by filling the 4f orbitals, while the Actinides fill the 5f orbitals. These elements are all metals, and many are radioactive. Their chemical properties are quite similar due to the relatively small difference in their electronic configurations.
5. Post-Transition Metals (p-block): A Borderline Case
Some elements in the p-block, particularly those near the 'staircase' that separates metals from nonmetals, exhibit properties intermediate between those of metals and nonmetals. These elements, such as aluminum (Al), tin (Sn), and lead (Pb), are often referred to as post-transition metals. They are less reactive than the transition metals and have some properties characteristic of metalloids.
6. Metalloids: Bridging the Gap Between Metals and Nonmetals
The metalloids or semimetals (boron, silicon, germanium, arsenic, antimony, tellurium, and polonium) lie along a diagonal line separating metals and nonmetals in the periodic table. They possess intermediate properties between metals and nonmetals and are therefore not strictly categorized as metals. Their conductivity, for example, can vary greatly depending on temperature or other factors.
The "Why" Behind Metallic Element Location
The arrangement of metals in the periodic table is directly linked to their electronic configurations and the forces governing atomic interactions.
-
Electropositive Nature: Metals tend to have low electronegativity; they readily lose electrons to achieve a stable electron configuration. This is reflected in their location on the left side of the periodic table, where elements generally have lower electronegativity.
-
Electron Sea Model: Metallic bonding, which is responsible for many of the characteristic metallic properties, involves a "sea" of delocalized electrons surrounding a lattice of positively charged metal ions. The ease of electron delocalization contributes to high electrical and thermal conductivity. Elements with this type of bonding are found concentrated in the left and central regions of the periodic table.
-
Atomic Size and Shielding Effect: As you move down a group, atomic size increases due to the addition of electron shells. This increased size and the shielding effect of inner electrons lead to a decrease in the effective nuclear charge, making it easier for the outermost electrons to be lost. This is why reactivity generally increases down a group in the alkali and alkaline earth metals.
Applications of Metals: A Vast and Diverse Landscape
The location of metals in the periodic table helps predict their properties and subsequently, their applications. This leads to a vast array of uses across various fields:
-
Construction and Engineering: Iron (Fe), steel (an alloy of iron and carbon), aluminum (Al), and other metals are essential building materials in construction, infrastructure development, and manufacturing.
-
Electronics: Copper (Cu), silver (Ag), gold (Au), and other metals are vital in the electronics industry, used in wiring, circuits, and electronic components.
-
Transportation: Aluminum alloys are used in aircraft manufacturing for their lightweight and high strength. Steel is still widely used in automobiles and other vehicles.
-
Medicine: Titanium (Ti) is used in surgical implants due to its biocompatibility. Other metals have various applications in medical devices and treatments.
-
Catalysis: Platinum (Pt), palladium (Pd), and other transition metals are employed extensively as catalysts in various chemical processes and industrial applications.
-
Energy: Rare earth elements are critical in the production of magnets and other components used in renewable energy technologies, such as wind turbines and electric vehicles.
Conclusion: The Periodic Table – A Map to Metallic Properties
The periodic table is far more than a simple list of elements; it’s a powerful tool providing insights into the relationships between elements and their properties. The location of metals within this structure provides valuable information about their electronic configurations, bonding behavior, and consequent applications. Understanding this organization is key to comprehending the fundamental principles of chemistry and the wide-ranging applications of metallic elements in our world. From the highly reactive alkali metals to the versatile transition metals and the rare earth elements crucial for modern technologies, each metal's position on the periodic table reveals a story of its unique properties and importance. This detailed exploration offers a comprehensive understanding of where metals are found in the periodic table and why their location is so significant in determining their characteristics and uses.
Latest Posts
Latest Posts
-
The Demand Curve For A Monopoly Is
Mar 04, 2025
-
Straight Line Is How Many Degrees
Mar 04, 2025
-
How Is Element Different From Compound
Mar 04, 2025
-
Is Light Energy Potential Or Kinetic
Mar 04, 2025
-
How Do I Find The Perimeter Of A Triangle
Mar 04, 2025
Related Post
Thank you for visiting our website which covers about Where Are The Metals In The Periodic Table Found . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
