How Is Element Different From Compound
Juapaving
Mar 04, 2025 · 6 min read
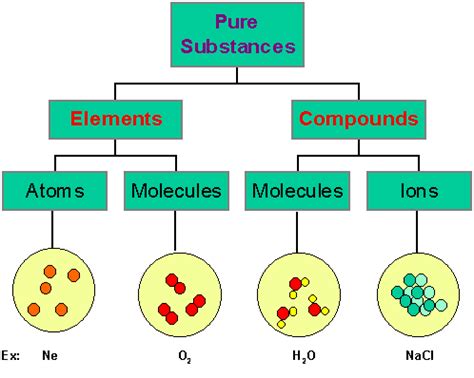
Table of Contents
How is an Element Different from a Compound? A Deep Dive into Chemical Structures
Understanding the fundamental building blocks of matter is crucial to grasping the complexities of chemistry. At the heart of this understanding lies the distinction between elements and compounds. While both are forms of matter, their composition and properties differ significantly. This article delves deep into the differences between elements and compounds, exploring their characteristics, examples, and the importance of this distinction in various scientific fields.
Defining Elements: The Fundamental Building Blocks
An element is a pure substance consisting entirely of one type of atom. This means all the atoms within an element share the same number of protons in their nucleus, which defines the element's atomic number. Elements are the simplest form of matter that cannot be broken down into simpler substances by chemical means. There are currently 118 known elements, each represented by a unique symbol on the periodic table.
Key Characteristics of Elements:
- Unique Atomic Number: The defining characteristic of an element is its atomic number, representing the number of protons in its nucleus. This number dictates the element's chemical properties and its position on the periodic table.
- Cannot be Broken Down: Elements cannot be broken down into simpler substances through chemical reactions. They represent the fundamental building blocks of all matter.
- Specific Physical and Chemical Properties: Each element possesses a unique set of physical properties (like melting point, boiling point, density) and chemical properties (like reactivity, oxidation states). These properties are determined by the element's atomic structure and electron configuration.
- Represented by Symbols: Elements are represented by one- or two-letter symbols, often derived from their Latin or English names (e.g., H for Hydrogen, O for Oxygen, Fe for Iron).
Examples of Elements:
The periodic table provides a comprehensive list of all known elements. Some common examples include:
- Hydrogen (H): The lightest and most abundant element in the universe.
- Oxygen (O): Essential for respiration and a major component of water.
- Carbon (C): The basis of all organic life and a key component of many materials.
- Iron (Fe): A vital element in many biological processes and used extensively in construction.
- Gold (Au): A highly valuable and inert metal.
Defining Compounds: Combinations of Elements
A compound is a pure substance composed of two or more different elements chemically bonded together in fixed proportions. These bonds can be ionic (involving the transfer of electrons) or covalent (involving the sharing of electrons). The properties of a compound are often vastly different from the properties of its constituent elements.
Key Characteristics of Compounds:
- Fixed Composition: Compounds always have a fixed ratio of elements. This ratio is determined by the chemical formula of the compound. For example, water (H₂O) always has a 2:1 ratio of hydrogen to oxygen atoms.
- Can be Broken Down: Compounds can be broken down into simpler substances (their constituent elements) through chemical reactions, such as electrolysis or combustion.
- Unique Properties: The properties of a compound are different from the properties of the elements it is composed of. For instance, sodium (Na) is a highly reactive metal and chlorine (Cl) is a toxic gas, but their compound, sodium chloride (NaCl), or table salt, is a relatively inert, crystalline solid.
- Represented by Formulas: Compounds are represented by chemical formulas that indicate the types and numbers of atoms present in a molecule or formula unit.
Examples of Compounds:
Numerous compounds exist, ranging from simple molecules to complex macromolecules. Examples include:
- Water (H₂O): A vital compound for life, consisting of hydrogen and oxygen.
- Carbon Dioxide (CO₂): A greenhouse gas produced by respiration and combustion.
- Sodium Chloride (NaCl): Table salt, an ionic compound formed from sodium and chlorine.
- Glucose (C₆H₁₂O₆): A simple sugar, an organic compound crucial for energy production.
- Sulfuric Acid (H₂SO₄): A strong acid used in many industrial processes.
The Crucial Differences Between Elements and Compounds: A Comparative Table
| Feature | Element | Compound |
|---|---|---|
| Composition | One type of atom | Two or more different elements chemically bonded |
| Purity | Pure substance | Pure substance |
| Breakdown | Cannot be broken down chemically | Can be broken down chemically into elements |
| Properties | Unique properties | Properties different from constituent elements |
| Representation | Symbol (e.g., H, O, Fe) | Chemical formula (e.g., H₂O, NaCl, CO₂) |
| Formation | Found naturally or produced through nuclear reactions | Formed through chemical reactions |
Illustrative Examples Highlighting the Differences
Let's consider some specific examples to further clarify the distinctions:
Example 1: Water (H₂O)
Water is a compound composed of two elements: hydrogen and oxygen. Hydrogen is a highly flammable gas, and oxygen supports combustion. However, water itself is neither flammable nor a strong oxidizing agent. This illustrates how the properties of a compound are distinct from those of its constituent elements. Water can be broken down into its constituent elements through electrolysis, a chemical process.
Example 2: Iron Oxide (Fe₂O₃)
Iron (Fe) is a strong, metallic element, while oxygen (O) is a gas essential for respiration. When these elements combine to form iron oxide (rust), the resulting compound is a brittle, reddish-brown solid with completely different properties than its components. The chemical reaction of iron and oxygen to form rust is a clear example of compound formation.
The Importance of Distinguishing Elements and Compounds
The distinction between elements and compounds is fundamental to various scientific disciplines:
- Chemistry: Understanding this distinction is central to comprehending chemical reactions, bonding, and the properties of matter.
- Biology: The composition of living organisms relies heavily on specific elements and compounds, highlighting their crucial roles in biological processes.
- Materials Science: The properties of materials, whether natural or synthetic, are directly related to their elemental and compound composition.
- Geology: The composition of rocks and minerals reflects the elements and compounds present in the Earth's crust.
Beyond Elements and Compounds: Mixtures
It's important to note that matter also exists as mixtures, which are combinations of substances that are not chemically bonded. Mixtures can be homogeneous (uniform throughout, like saltwater) or heterogeneous (non-uniform, like sand and water). Unlike compounds, mixtures can be separated into their components through physical methods, such as filtration or distillation. Understanding mixtures, alongside elements and compounds, provides a comprehensive understanding of matter's diverse forms.
Conclusion: A Foundation for Chemical Understanding
The difference between elements and compounds is a cornerstone concept in chemistry and related fields. Elements, the fundamental building blocks of matter, are pure substances consisting of only one type of atom. Compounds, on the other hand, are formed through the chemical combination of two or more elements in fixed proportions, exhibiting properties distinct from their constituent elements. Understanding this fundamental distinction is crucial for comprehending the composition, properties, and behavior of matter in diverse contexts. This knowledge provides a strong foundation for further exploration into the fascinating world of chemistry and its myriad applications.
Latest Posts
Latest Posts
-
What Is The Si Unit Of Momentum
Mar 05, 2025
-
What 2 Planets Have No Moons
Mar 05, 2025
-
Which Of The Following Are Components Of A Nucleotide
Mar 05, 2025
-
What Is A 7 8 Grade Percentage
Mar 05, 2025
-
What Vitamin Is Neither Fat Nor Water Soluble
Mar 05, 2025
Related Post
Thank you for visiting our website which covers about How Is Element Different From Compound . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
