What Is The Electron Configuration For I
Juapaving
Mar 18, 2025 · 6 min read
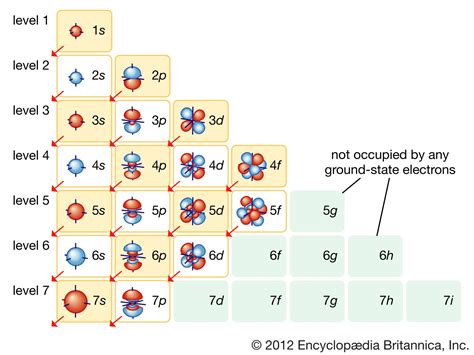
Table of Contents
What is the Electron Configuration for Iodine? A Deep Dive into Atomic Structure
Iodine, a fascinating element with a rich history and crucial role in human biology, holds a unique place in the periodic table. Understanding its electron configuration is key to unlocking its properties and behavior. This comprehensive article will explore the electron configuration of iodine (I), delving into the underlying principles of atomic structure and exploring the implications of this configuration on iodine's chemical reactivity and physical characteristics.
Understanding Electron Configuration
Before we delve into the specifics of iodine's electron configuration, let's establish a foundational understanding of what electron configuration represents. An element's electron configuration describes how electrons are distributed among the various energy levels and sublevels within an atom. These electrons occupy orbitals, regions of space around the nucleus where there's a high probability of finding an electron. The configuration follows specific rules dictated by quantum mechanics, namely the Aufbau principle, Hund's rule, and the Pauli exclusion principle.
- Aufbau principle: Electrons fill the lowest energy levels first.
- Hund's rule: Electrons individually occupy each orbital within a subshell before pairing up.
- Pauli exclusion principle: Each orbital can hold a maximum of two electrons with opposite spins.
These principles guide us in predicting the electron configuration of any element, including iodine.
Determining the Electron Configuration of Iodine (I)
Iodine (I) has an atomic number of 53, meaning it has 53 protons and, in a neutral atom, 53 electrons. To determine its electron configuration, we systematically fill the electron shells and subshells according to the Aufbau principle.
The order of filling is typically represented as: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p...
Each subshell can hold a specific number of electrons:
- s subshell: holds a maximum of 2 electrons
- p subshell: holds a maximum of 6 electrons
- d subshell: holds a maximum of 10 electrons
- f subshell: holds a maximum of 14 electrons
Following this order, the complete electron configuration for iodine (I) is: 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁶5s²4d¹⁰5p⁵.
This can also be written in a shorthand notation using the noble gas configuration, referencing the nearest noble gas with a lower atomic number (Krypton, Kr): [Kr] 5s²4d¹⁰5p⁵. This shorthand notation simplifies the representation while still conveying all the essential information.
The Significance of Iodine's Outermost Electrons
The outermost electrons, those in the highest energy level (valence electrons), are particularly important because they determine an element's chemical reactivity. In iodine's case, these are the 5s² and 5p⁵ electrons. The presence of seven valence electrons (2 + 5) makes iodine a highly reactive element, readily gaining one electron to achieve a stable octet (eight electrons in its outermost shell) and forming the iodide ion (I⁻).
Iodine's Chemical Behavior and Electron Configuration
Iodine's electron configuration directly influences its chemical properties. Its tendency to gain an electron to complete its octet explains its strong oxidizing power. It readily reacts with metals to form ionic compounds, where it exists as the iodide anion (I⁻). For example, the reaction between sodium (Na) and iodine (I₂) produces sodium iodide (NaI):
2Na(s) + I₂(s) → 2NaI(s)
In this reaction, sodium readily donates one electron to each iodine atom, forming Na⁺ and I⁻ ions, which are electrostatically attracted to each other.
Iodine also participates in covalent bonding, sharing electrons with other nonmetals to form molecules. For instance, iodine forms diatomic molecules (I₂) in its elemental form, where two iodine atoms share electrons to achieve a more stable configuration.
Iodine's Physical Properties and their Link to Electron Configuration
The electron configuration of iodine also influences its physical properties. Iodine is a solid at room temperature, exhibiting a dark purplish-black, crystalline appearance. This is attributed to the strong intermolecular forces (van der Waals forces) between its diatomic molecules. These forces arise from the interaction of electrons within the molecules, a consequence of its electron configuration.
Iodine's relatively low melting and boiling points compared to other halogens are partly due to the weaker intermolecular forces present between iodine molecules. The larger size of the iodine atom compared to other halogens results in a lower effective nuclear charge, leading to weaker van der Waals forces.
Iodine exhibits sublimation, meaning it can transition directly from solid to gas phase without passing through the liquid phase. This phenomenon is again related to the relatively weak intermolecular forces.
Iodine's Biological Role and the Importance of Electron Configuration
Iodine plays a vital biological role, primarily as a component of thyroid hormones (thyroxine (T4) and triiodothyronine (T3)). These hormones regulate metabolism, growth, and development. The ability of iodine to readily form ionic bonds is crucial for its incorporation into these hormones. The iodide ion (I⁻) is actively transported into the thyroid gland where it undergoes oxidation and is incorporated into the thyroglobulin protein, eventually forming the thyroid hormones. The precise electronic structure of iodine, specifically its ability to easily gain an electron, is fundamental to this process.
Excited States of Iodine
While the ground state electron configuration is the most stable, iodine atoms can also exist in excited states. In these states, one or more electrons absorb energy and jump to higher energy levels. These excited states are temporary, and the electrons eventually return to their ground state, emitting energy in the process. This energy emission can manifest as the characteristic absorption or emission spectra of iodine, which can be used for analytical purposes.
Isotopes of Iodine and their Electron Configuration
Iodine has several isotopes, atoms with the same number of protons but different numbers of neutrons. The most common isotope is Iodine-127 (¹²⁷I). However, other isotopes exist, some of which are radioactive. Regardless of the isotope, the electron configuration remains the same for a neutral iodine atom because the number of electrons is determined by the number of protons (atomic number), which does not change with different isotopes. Only the mass number (protons + neutrons) is altered.
Conclusion: The Intricate Relationship between Electron Configuration and Iodine's Properties
The electron configuration of iodine ([Kr] 5s²4d¹⁰5p⁵) provides a fundamental understanding of its various properties. From its chemical reactivity and ability to form ionic and covalent compounds, to its physical characteristics such as sublimation and its crucial biological role in thyroid hormone production, the arrangement of electrons dictates its behaviour. By understanding its electron configuration, we gain profound insights into the fascinating world of this essential element. This knowledge is pivotal in various fields, from chemistry and physics to biology and medicine, highlighting the power of atomic structure in explaining the world around us. Further research into iodine's interactions and the implications of its electron configuration continues to reveal new and exciting possibilities.
Latest Posts
Latest Posts
-
Is Density An Intensive Or Extensive Property
Mar 18, 2025
-
Words That End In The Letter E
Mar 18, 2025
-
What Is The Formula For The Chlorate Ion
Mar 18, 2025
-
Chemical Reaction Of Iron And Water
Mar 18, 2025
-
Write The Prime Factorization Of 27
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about What Is The Electron Configuration For I . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
