What Is The Formula For The Chlorate Ion
Juapaving
Mar 18, 2025 · 6 min read
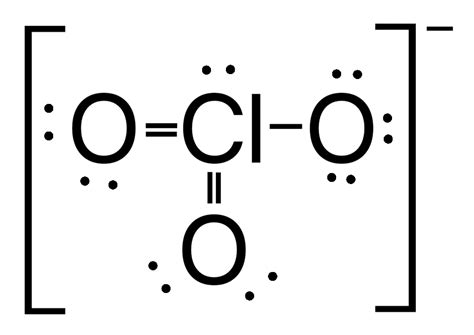
Table of Contents
What is the Formula for the Chlorate Ion? A Deep Dive into Chlorate Chemistry
The chlorate ion, a fascinating and versatile chemical species, plays a significant role in various chemical processes and industrial applications. Understanding its formula, structure, properties, and reactions is crucial for anyone working with chlorine-based compounds. This comprehensive article will delve into the specifics of the chlorate ion, exploring its formula, properties, preparation, uses, and safety considerations.
The Formula: ClO₃⁻
The chemical formula for the chlorate ion is ClO₃⁻. This formula indicates that the ion consists of one chlorine atom (Cl) and three oxygen atoms (O), carrying a single negative charge (-). This negative charge arises from the unequal sharing of electrons between the chlorine and oxygen atoms, resulting in an overall excess of electrons on the ion.
Understanding the Structure: A Molecular Perspective
The structure of the chlorate ion is crucial to understanding its properties and reactivity. The chlorine atom is at the center, surrounded by three oxygen atoms. These oxygen atoms are arranged in a trigonal pyramidal geometry, meaning they are not in a flat plane but rather form a pyramid with the chlorine atom at the apex. This geometry is due to the presence of electron lone pairs on the chlorine atom, which repel the bonding pairs of electrons, resulting in the pyramidal arrangement.
This trigonal pyramidal shape is critical in determining the ion's reactivity. The unsymmetrical distribution of charge within the ion contributes to its oxidizing power.
Resonance Structures: A More Complex Picture
The actual structure of the chlorate ion is best described as a resonance hybrid. This means that the actual structure is a weighted average of multiple contributing structures, where the double bonds between chlorine and oxygen are delocalized. While it’s often simplified to a single structure with one Cl=O double bond and two Cl-O single bonds, the reality is more nuanced. The electron density is evenly distributed amongst the three oxygen atoms, making each Cl-O bond essentially identical in bond length and strength.
This resonance stabilization contributes to the relative stability of the chlorate ion compared to some other oxychlorine anions.
Properties of the Chlorate Ion: A Closer Look
The chlorate ion possesses several key properties that make it both useful and potentially hazardous.
Oxidation State of Chlorine
The oxidation state of chlorine in the chlorate ion is +5. This high oxidation state makes the chlorate ion a strong oxidizing agent. This means it readily accepts electrons from other substances, leading to its involvement in redox reactions.
Solubility
Chlorate salts, which contain the chlorate ion, are generally highly soluble in water. This high solubility is beneficial for many industrial applications where solutions of chlorate ions are needed.
Thermal Stability
Chlorate salts have moderate thermal stability, meaning they decompose upon heating to higher temperatures. This decomposition often leads to the formation of chlorides, oxygen gas, and sometimes other byproducts. The exact decomposition products and temperature depend on the specific counterion of the salt and any other conditions present. This property is critical to consider for storage and handling safety.
Reactivity: A Powerful Oxidizer
The high oxidation state of chlorine in ClO₃⁻ lends to its significant oxidizing power. It reacts readily with various reducing agents, leading to its use in various applications involving oxidation reactions. This reactivity necessitates careful handling and storage.
Preparation of Chlorates: Industrial and Laboratory Methods
Chlorates are primarily produced industrially using the electrolysis of chloride solutions. In this process, an aqueous solution of sodium chloride (NaCl) is electrolyzed, leading to the formation of chlorate ions at the anode. The process is complex and involves multiple intermediate steps, including the formation of hypochlorite ions (ClO⁻) as a precursor to chlorate formation.
Laboratory-scale preparation typically involves the disproportionation of hypochlorites in a controlled environment. This reaction involves converting hypochlorite ions into both chlorate and chloride ions, carefully balancing the conditions to maximize chlorate production. The specific methods employed vary based on the desired chlorate salt and the available equipment.
Uses of Chlorate Ion and its Salts: A Versatile Compound
Chlorate ions and their salts (e.g., potassium chlorate, sodium chlorate) find wide-ranging applications in various industries and fields.
Herbicides and Pesticides
Chlorate salts, especially sodium chlorate, are commonly used as herbicides. Their effectiveness stems from their ability to disrupt plant metabolic processes, leading to their death. However, due to environmental concerns, the use of chlorate herbicides is increasingly regulated.
Oxidizing Agents in Industry
The strong oxidizing power of chlorate ions makes them suitable for various industrial processes requiring oxidation. They are used as oxidizers in:
- Matches: Potassium chlorate was historically used in matches to provide the oxidizing agent needed for combustion.
- Explosives: Although less common now, some explosives incorporate chlorates due to their oxidizing properties.
- Textile industry: Chlorates find use in bleaching and other oxidation-related processes in the textile industry.
- Pyrotechnics: Chlorates contribute to the vibrant colors and effects in fireworks, providing oxygen for the combustion of other pyrotechnic materials.
Other Applications
Chlorate ions and their salts have found niche applications in other areas, including:
- Water treatment: In some specialized water treatment processes, chlorates may be employed as an oxidizing agent.
- Chemical synthesis: Chlorates can act as a reagent in specific chemical syntheses, providing a source of oxygen for oxidation reactions.
Safety Considerations: Handling Chlorates Responsibly
Chlorate salts present some significant safety hazards due to their oxidizing power and potential for decomposition. Appropriate safety measures must always be employed during handling and storage:
Fire Hazard
Chlorates are strong oxidizers, posing a significant fire hazard. They can readily ignite combustible materials, so keeping them away from flammable substances is crucial. Mixing chlorates with organic materials or easily oxidized substances can lead to spontaneous combustion or explosions.
Toxicity
Certain chlorate salts exhibit toxicity, causing harmful effects upon ingestion or inhalation. Appropriate personal protective equipment (PPE), such as gloves and eye protection, is necessary during handling. Proper ventilation is also essential to prevent inhalation of chlorate dust or fumes.
Decomposition
The thermal decomposition of chlorates produces oxygen gas and potentially toxic chlorine-containing compounds. Heating chlorates should only be done under strictly controlled conditions, and appropriate ventilation is necessary to prevent the accumulation of potentially harmful decomposition products.
Conclusion: A Powerful Ion with Broad Applications
The chlorate ion (ClO₃⁻), with its unique formula, structure, and properties, plays a vital role in various chemical processes and industrial applications. Its strong oxidizing power makes it a versatile compound used in diverse areas, from herbicides to pyrotechnics. However, the inherent hazards associated with its reactivity and toxicity necessitate careful handling and appropriate safety measures. Understanding the formula and properties of the chlorate ion is paramount for safe and responsible use in any context. Further research continues to explore new applications and optimize safety protocols surrounding this remarkable chemical species. Its role in industry and its potential for new applications continue to make the chlorate ion a subject of ongoing interest and study.
Latest Posts
Latest Posts
-
How Many Elements Are In Water
Mar 18, 2025
-
What Is Meant By Translational Kinetic Energy
Mar 18, 2025
-
Is Salt A Compound Mixture Or Element
Mar 18, 2025
-
What Is The Lcm Of 24 And 14
Mar 18, 2025
-
Highest Common Factor Of 2 And 8
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about What Is The Formula For The Chlorate Ion . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
