Is Density An Intensive Or Extensive Property
Juapaving
Mar 18, 2025 · 6 min read
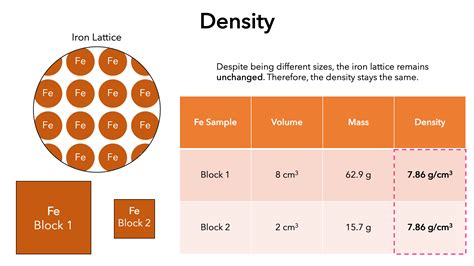
Table of Contents
Is Density an Intensive or Extensive Property? A Deep Dive
Density, a fundamental concept in physics and chemistry, often sparks confusion regarding its classification as an intensive or extensive property. This comprehensive guide will delve into the intricacies of density, exploring its definition, calculation, and ultimately, definitively answering whether density is intensive or extensive. We'll also explore the implications of this classification and how it relates to other properties of matter.
Understanding Intensive and Extensive Properties
Before classifying density, let's establish a clear understanding of the distinction between intensive and extensive properties. This crucial distinction underpins many aspects of physical science.
Extensive Properties: Dependent on Amount
Extensive properties are those that depend on the amount of matter present. If you double the amount of a substance, you double the value of its extensive properties. Examples include:
- Mass: The quantity of matter in an object. A larger object has a greater mass.
- Volume: The amount of space occupied by an object. A larger object occupies a greater volume.
- Length: A linear measurement of an object. A longer object has a greater length.
- Heat Capacity: The amount of heat required to raise the temperature of a substance by a certain degree. More substance, more heat needed.
Intensive Properties: Independent of Amount
Intensive properties, on the other hand, are independent of the amount of matter. No matter how much of a substance you have, its intensive properties remain the same. Examples include:
- Temperature: A measure of the average kinetic energy of the particles in a substance.
- Pressure: The force exerted per unit area.
- Density: The mass per unit volume.
- Boiling Point: The temperature at which a liquid boils.
- Melting Point: The temperature at which a solid melts.
Defining Density: Mass Divided by Volume
Density is defined as the mass of a substance per unit volume. Mathematically, it's represented as:
Density (ρ) = Mass (m) / Volume (V)
This simple equation is fundamental to understanding density's behavior. Let's explore this equation in the context of intensive versus extensive properties.
Is Density Intensive or Extensive? The Crucial Argument
Mass (m) and volume (V), as discussed earlier, are both extensive properties. However, their ratio—density—exhibits a different behavior. This is where the critical understanding comes into play.
Consider a block of iron. If you cut the block in half, you've halved its mass and halved its volume. While both mass and volume have changed, their ratio – the density – remains constant. This illustrates the defining characteristic of an intensive property: it is independent of the amount of material. Whether you have a small piece of iron or a large one, the density remains the same (assuming uniform composition and conditions).
Therefore, density is an intensive property.
Further Clarification: A Deeper Look at the Ratio
The ratio of two extensive properties can result in an intensive property. This is a key principle to remember. Other examples of this include:
- Concentration: The amount of solute per unit volume of solution (moles/liter). Both moles (amount of solute) and volume are extensive, but concentration is intensive.
- Specific Heat Capacity: The amount of heat required to raise the temperature of one unit mass of a substance by one degree. Both heat and mass are extensive, yet specific heat capacity is intensive.
These examples further solidify the concept that the ratio of two extensive properties can result in an intensive property.
Implications of Density's Intensive Nature
The fact that density is an intensive property has significant implications in various fields:
- Material Identification: Density is a crucial property used to identify unknown substances. Since it's intensive, a small sample is sufficient for identification.
- Fluid Mechanics: Density plays a pivotal role in understanding fluid behavior, buoyancy, and flow patterns. Its intensive nature simplifies calculations, as the density remains constant regardless of the volume of the fluid.
- Engineering and Design: Density is a critical factor in structural design and material selection. Engineers need to consider the density of materials to ensure structural integrity and efficiency.
- Geophysics and Geology: Density differences in Earth's layers are crucial in understanding plate tectonics and geophysical processes. The density of rocks and minerals helps in geological mapping and resource exploration.
- Chemistry: Density is extensively used in chemical analyses and calculations, particularly in determining the concentration of solutions and identifying unknown compounds.
Density Variations: Factors to Consider
While density is inherently intensive, several factors can influence the measured density of a substance:
- Temperature: Temperature changes affect the volume of a substance, thus affecting its density. Generally, substances expand with increasing temperature, leading to a decrease in density.
- Pressure: Pressure changes can compress a substance, altering its volume and consequently its density. Increased pressure usually leads to increased density.
- Composition: For mixtures and solutions, the density will vary depending on the composition and proportions of the components. This makes density a useful tool for determining composition.
- Phase Changes: The density of a substance changes significantly during phase transitions (solid to liquid to gas). For example, ice is less dense than liquid water, which is why ice floats.
Understanding these factors is crucial for accurate density measurements and interpretations.
Practical Applications and Examples
Let's illustrate the concept of density's intensive nature with some practical examples:
- Gold: The density of gold is approximately 19.3 g/cm³. This value remains the same whether you have a gold nugget or a gold bar; the density doesn't depend on the amount.
- Water: The density of water at 4°C is approximately 1 g/cm³. This value remains constant for a drop of water, a glass of water, or a swimming pool full of water.
- Air: The density of air varies with altitude and temperature. However, at a specific temperature and pressure, the density will be the same regardless of the volume of air sampled.
These examples highlight the importance of specifying the conditions (temperature and pressure) when reporting density values, especially for gases and liquids, where density is more susceptible to changes.
Conclusion: Density's Unwavering Intensity
In conclusion, density, defined as the mass per unit volume, is unequivocally an intensive property. While mass and volume are extensive, their ratio—density—remains constant irrespective of the amount of substance present. This fundamental characteristic makes density an invaluable tool in various scientific and engineering disciplines, enabling material identification, fluid dynamics analysis, and much more. Understanding the intensive nature of density and its relation to temperature, pressure, and composition is critical for accurate measurements and meaningful interpretations. By grasping this concept, we can unlock a deeper understanding of the physical properties of matter and the world around us.
Latest Posts
Latest Posts
-
Least Common Multiple Of 27 And 45
Mar 18, 2025
-
How Do You Turn Gas Into A Liquid
Mar 18, 2025
-
A Push Or Pull Is Called
Mar 18, 2025
-
What Are The Prime Factors Of 87
Mar 18, 2025
-
Are Diagonals Of A Parallelogram Perpendicular
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Is Density An Intensive Or Extensive Property . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
