What Is Not A Pure Substance
Juapaving
Mar 26, 2025 · 6 min read
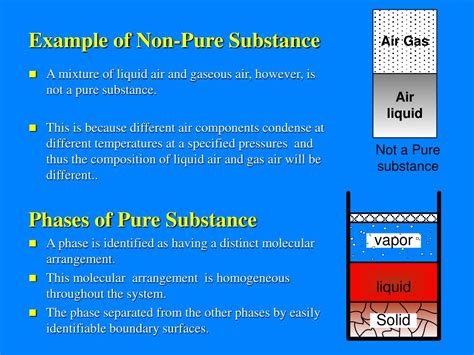
Table of Contents
What is NOT a Pure Substance? A Deep Dive into Mixtures and Their Properties
Understanding the difference between pure substances and mixtures is fundamental to chemistry. While a pure substance consists of only one type of atom or molecule, a mixture contains two or more different substances that are not chemically bonded. This article will explore the fascinating world of mixtures, delving into their various types, properties, and everyday examples. We'll also clarify common misconceptions and highlight the key characteristics that distinguish mixtures from pure substances.
Defining a Pure Substance: The Baseline
Before we dive into what isn't a pure substance, let's briefly define what is. A pure substance has a fixed chemical composition and consistent properties throughout. This means that no matter where you sample the substance from, its chemical makeup and physical properties (like melting point, boiling point, density) will remain the same. Examples include elements (like gold, oxygen, or iron) and compounds (like water, salt, or sugar). Elements are substances composed of only one type of atom, while compounds are formed by the chemical combination of two or more elements in a fixed ratio.
The World of Mixtures: A Diverse Landscape
Mixtures, in contrast, are physical combinations of two or more substances. These substances retain their individual chemical identities and are not chemically bonded. The key characteristic of a mixture is that its composition can vary. You can have a mixture with a high concentration of one substance and a low concentration of another, and this ratio is not fixed. This variability in composition leads to a range of properties that depend on the relative amounts of each component.
Types of Mixtures: Homogeneous vs. Heterogeneous
Mixtures are broadly classified into two categories: homogeneous and heterogeneous.
1. Homogeneous Mixtures: These mixtures have a uniform composition throughout. This means that the different components are evenly distributed at a microscopic level, and you can't visually distinguish one component from another. Examples include:
- Solutions: These are homogeneous mixtures where one substance (the solute) is dissolved in another (the solvent). Examples include saltwater (salt dissolved in water), sugar water, and air (oxygen, nitrogen, and other gases dissolved in each other).
- Alloys: These are homogeneous mixtures of metals. Examples include brass (copper and zinc), bronze (copper and tin), and steel (iron and carbon).
2. Heterogeneous Mixtures: In these mixtures, the components are not uniformly distributed. You can visually distinguish the different parts of the mixture. Examples include:
- Suspensions: These are mixtures where solid particles are dispersed in a liquid but do not dissolve. The particles are relatively large and will settle out over time if left undisturbed. Examples include muddy water, sand in water, and some paints.
- Colloids: These are mixtures where particles are dispersed in a medium, but they are smaller than in suspensions and do not settle out easily. They exhibit the Tyndall effect, scattering light. Examples include milk, fog, and mayonnaise.
- Mechanical Mixtures: These are mixtures where the different components are easily visible. Examples include a salad, a bowl of cereal, and soil.
Properties of Mixtures: Variable and Dependent
The properties of a mixture are determined by the properties of its components and their relative amounts. Unlike pure substances, mixtures don't have a fixed melting point or boiling point. Instead, they typically melt and boil over a range of temperatures. This is because the different components have different melting and boiling points. The density of a mixture also depends on the densities of its components and their proportions.
Other properties, such as color, texture, and taste, can also vary significantly depending on the mixture's composition. For example, the color of a paint mixture depends on the colors and amounts of pigments used, while the taste of a fruit salad depends on the types and ripeness of the fruits.
Distinguishing Mixtures from Pure Substances: Key Differences
The following table summarizes the key differences between pure substances and mixtures:
| Feature | Pure Substance | Mixture |
|---|---|---|
| Composition | Fixed and constant | Variable |
| Properties | Constant and consistent | Variable and dependent on composition |
| Melting Point | Sharp, defined | Range of temperatures |
| Boiling Point | Sharp, defined | Range of temperatures |
| Separation | Cannot be separated physically | Can be separated by physical methods |
| Examples | Water (H₂O), Gold (Au), Oxygen (O₂) | Air, saltwater, salad |
Common Misconceptions about Mixtures
Let's address some frequently held misconceptions regarding mixtures:
-
Misconception 1: All mixtures are visibly heterogeneous. This is incorrect. Homogeneous mixtures, like saltwater or air, have a uniform composition throughout and are not visibly heterogeneous.
-
Misconception 2: Mixtures cannot be separated. This is also false. Many mixtures can be separated using various physical methods like filtration, distillation, evaporation, chromatography, and magnetism. The choice of separation technique depends on the properties of the components in the mixture.
-
Misconception 3: A mixture always involves a liquid. This is inaccurate. Mixtures can exist in all three states of matter—solid, liquid, and gas. Examples include solid alloys (brass), liquid solutions (saltwater), and gaseous mixtures (air).
Separating Mixtures: Techniques and Applications
The ability to separate mixtures is crucial in various fields, including chemistry, engineering, and environmental science. Different separation techniques exploit the unique properties of the components in a mixture:
-
Filtration: This method separates solids from liquids using a porous material like filter paper. This is effective for separating mixtures like sand and water.
-
Distillation: This technique separates liquids based on their boiling points. The liquid with the lower boiling point will vaporize first and can be collected separately. This is commonly used to purify water or separate components of crude oil.
-
Evaporation: This method separates a dissolved solid from a liquid by allowing the liquid to evaporate, leaving the solid behind. This is commonly used to obtain salt from saltwater.
-
Chromatography: This technique separates components of a mixture based on their different affinities for a stationary phase (e.g., paper or silica gel) and a mobile phase (e.g., a solvent). This method is used extensively in analytical chemistry.
-
Decantation: This simple technique involves carefully pouring off the liquid from a sediment or precipitate, leaving the solid behind.
-
Magnetism: This method separates magnetic materials from non-magnetic materials using a magnet. This is useful for separating iron filings from sand.
-
Centrifugation: This involves spinning a mixture at high speeds, causing denser components to settle at the bottom. This is widely used in separating blood components or sediment in wastewater treatment.
Examples of Mixtures in Everyday Life
Mixtures are ubiquitous in our daily lives. From the food we eat to the air we breathe, we encounter countless examples of mixtures.
-
Food: Most foods are mixtures. A salad is a heterogeneous mixture of vegetables, a cake is a complex mixture of flour, sugar, eggs, and other ingredients, and even milk is a colloid.
-
Air: The air we breathe is a homogeneous mixture of gases, primarily nitrogen and oxygen.
-
Seawater: This is a homogeneous solution of water and various salts.
-
Soil: This is a heterogeneous mixture of minerals, organic matter, water, and air.
-
Household cleaning products: Many cleaning products are mixtures designed for specific cleaning tasks.
Conclusion: Understanding Mixtures – A Crucial Skill
Understanding the concept of mixtures is crucial for comprehending the world around us. The ability to distinguish between pure substances and mixtures, to classify mixtures into different types, and to apply appropriate separation techniques is fundamental in various scientific and technological fields. This article has provided a comprehensive overview of the topic, highlighting key differences, examples, and applications. By grasping these concepts, you can enhance your understanding of chemistry and the diverse nature of matter.
Latest Posts
Latest Posts
-
An Acid Is Defined As A Substance That
Mar 29, 2025
-
Correctly Label The Following Parts Of The Male Reproductive System
Mar 29, 2025
-
Label The Diagram Of The Male Reproductive System
Mar 29, 2025
-
Moment Of Inertia Of A Rectangular Plate
Mar 29, 2025
-
2 Lines Cut By A Transversal
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about What Is Not A Pure Substance . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
