An Acid Is Defined As A Substance That
Juapaving
Mar 29, 2025 · 6 min read
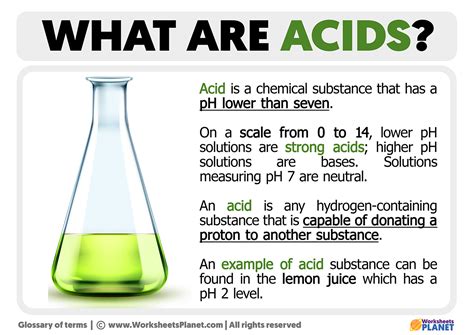
Table of Contents
- An Acid Is Defined As A Substance That
- Table of Contents
- An Acid is Defined as a Substance That... Donates Protons! A Deep Dive into Acid Chemistry
- The Brønsted-Lowry Definition: The Proton Donor
- The Arrhenius Definition: An Older, More Limited Perspective
- The Lewis Definition: A Broader View of Acidity
- Properties of Acids
- Types of Acids
- Applications of Acids
- Safety Precautions when Handling Acids
- Conclusion: A Fundamental Concept in Chemistry
- Latest Posts
- Latest Posts
- Related Post
An Acid is Defined as a Substance That... Donates Protons! A Deep Dive into Acid Chemistry
Acids. The word conjures images of corrosive liquids, burning sensations, and perhaps even a chemistry lab experiment gone wrong. But acids are far more nuanced and fundamental than that simple picture suggests. Understanding what defines an acid, its properties, and its role in the world around us is crucial, not only for scientific understanding, but also for everyday life. This comprehensive guide will delve deep into the definition of an acid, exploring its various theories, properties, and applications.
The Brønsted-Lowry Definition: The Proton Donor
The most commonly used definition of an acid is the Brønsted-Lowry definition. This definition, proposed independently by Johannes Nicolaus Brønsted and Thomas Martin Lowry in 1923, defines an acid as a substance that donates a proton (H⁺). A proton, in this context, is a hydrogen ion – essentially a hydrogen atom that has lost its electron, leaving only the positively charged nucleus.
This definition is remarkably powerful because it expands the concept of an acid beyond just substances dissolved in water. Many reactions involving proton transfer occur in non-aqueous solvents, and the Brønsted-Lowry definition elegantly encompasses these scenarios.
Key features of the Brønsted-Lowry definition:
- Proton donation: The central tenet is the ability to donate a proton. This donation occurs through a chemical reaction, often involving the breaking of a bond.
- Conjugate acid-base pairs: When an acid donates a proton, it forms its conjugate base. The conjugate base is the species remaining after the proton has been donated. Simultaneously, the proton acceptor (a base) becomes its conjugate acid. This relationship creates conjugate acid-base pairs, which are crucial for understanding acid-base equilibrium.
- Applicability: The Brønsted-Lowry definition is applicable across a wide range of solvents and reaction conditions.
Example: Consider the reaction between hydrochloric acid (HCl) and water (H₂O):
HCl(aq) + H₂O(l) ⇌ H₃O⁺(aq) + Cl⁻(aq)
In this reaction, HCl acts as the acid, donating a proton to H₂O. H₂O acts as the base, accepting the proton. The resulting hydronium ion (H₃O⁺) is the conjugate acid of water, and the chloride ion (Cl⁻) is the conjugate base of HCl.
The Arrhenius Definition: An Older, More Limited Perspective
Before the Brønsted-Lowry definition, the most prevalent was the Arrhenius definition, proposed by Svante Arrhenius in 1884. This definition defines an acid as a substance that increases the concentration of hydrogen ions (H⁺) when dissolved in water.
While simpler than the Brønsted-Lowry definition, the Arrhenius definition has significant limitations:
- Water-centric: It is strictly limited to aqueous solutions. Reactions in non-aqueous solvents are not considered.
- Oversimplification: It doesn't explicitly describe the proton transfer mechanism, focusing solely on the outcome—increased H⁺ concentration.
While historically important, the Arrhenius definition is less comprehensive and less frequently used than the Brønsted-Lowry definition in modern chemistry.
The Lewis Definition: A Broader View of Acidity
The Lewis definition, proposed by Gilbert N. Lewis in 1923, offers the broadest perspective on acidity. It defines an acid as a substance that accepts a pair of electrons. This definition encompasses a much wider range of substances than the Brønsted-Lowry definition, including those that don't possess a proton to donate.
Key aspects of the Lewis definition:
- Electron pair acceptance: The core concept is the acceptance of an electron pair, forming a coordinate covalent bond.
- Lewis acids and bases: Substances that donate electron pairs are known as Lewis bases, while those that accept them are Lewis acids.
- Expanding the scope: This definition includes many substances that are not traditionally considered acids based on the Brønsted-Lowry definition, such as boron trifluoride (BF₃) and aluminum chloride (AlCl₃).
Example: The reaction between boron trifluoride (BF₃) and ammonia (NH₃):
BF₃ + NH₃ → F₃B-NH₃
In this reaction, BF₃ acts as a Lewis acid, accepting an electron pair from the nitrogen atom in NH₃. NH₃ acts as a Lewis base, donating the electron pair.
Properties of Acids
Acids exhibit a range of characteristic properties:
- Sour taste: Many acids possess a distinctly sour taste (though caution should always be exercised – never taste unknown substances!).
- Reaction with metals: Many acids react with active metals like zinc and magnesium, producing hydrogen gas and a metal salt.
- pH less than 7: Aqueous solutions of acids have a pH less than 7, indicating a higher concentration of hydrogen ions.
- Reaction with bases: Acids react with bases in a process called neutralization, producing salt and water.
- Change indicator color: Acids change the color of certain indicators, such as litmus paper (turning it red) and phenolphthalein (remaining colorless).
- Electrical conductivity: Aqueous solutions of strong acids are good conductors of electricity due to the presence of ions.
Types of Acids
Acids are categorized in several ways:
- Strong vs. Weak Acids: Strong acids, like HCl and HNO₃, completely dissociate in water, releasing all their protons. Weak acids, like acetic acid (CH₃COOH), only partially dissociate, maintaining an equilibrium between the acid and its ions.
- Monoprotic, Diprotic, and Polyprotic Acids: Monoprotic acids donate one proton per molecule (e.g., HCl), diprotic acids donate two (e.g., H₂SO₄), and polyprotic acids donate more than two (e.g., H₃PO₄).
- Organic vs. Inorganic Acids: Organic acids contain carbon atoms (e.g., citric acid), while inorganic acids do not (e.g., sulfuric acid).
Applications of Acids
Acids play a crucial role in numerous applications across various fields:
- Industrial processes: Acids are essential in the manufacturing of various products, including fertilizers, plastics, and pharmaceuticals.
- Food and beverage industry: Acids like citric acid and acetic acid are used as preservatives and flavor enhancers.
- Cleaning agents: Acids are used in various cleaning products, such as toilet bowl cleaners and rust removers.
- Medical applications: Acids are used in medications and treatments for various conditions.
- Batteries: Acids are used as electrolytes in many types of batteries.
Safety Precautions when Handling Acids
Acids can be hazardous substances. Always exercise caution when handling acids, including:
- Wearing appropriate personal protective equipment (PPE): This includes gloves, eye protection, and lab coats.
- Working in a well-ventilated area: Many acids release fumes that can be harmful.
- Following proper disposal procedures: Acids should be disposed of according to local regulations.
- Never mix acids with other chemicals without proper knowledge: Unexpected and dangerous reactions can occur.
Conclusion: A Fundamental Concept in Chemistry
The definition of an acid, while seemingly simple, is a cornerstone of chemical understanding. From the simplest Arrhenius definition to the broader perspectives of Brønsted-Lowry and Lewis, the concept has evolved to encompass a vast array of substances and reactions. Understanding the properties and behaviors of acids is essential in various fields, ranging from industrial processes to everyday life. However, it's crucial to always remember the potential hazards associated with acids and to handle them with the utmost care and respect. This comprehensive overview provides a strong foundation for further exploration of this vital area of chemistry.
Latest Posts
Latest Posts
-
Is Spring Force A Conservative Force
Apr 02, 2025
-
A Group Of Sheep Is Called What
Apr 02, 2025
-
120 Sq Meters To Sq Ft
Apr 02, 2025
-
The Shared Endpoint Of Two Rays Is Called The
Apr 02, 2025
-
You Can Increase The Strength Of An Electromagnet By
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about An Acid Is Defined As A Substance That . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
