What Is Electron Configuration Of Potassium
Juapaving
Mar 31, 2025 · 6 min read
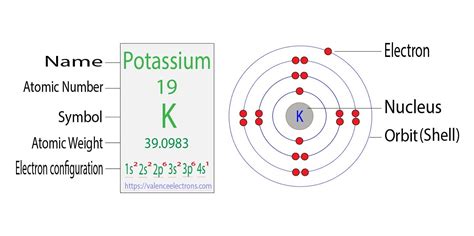
Table of Contents
What is the Electron Configuration of Potassium? A Deep Dive into Atomic Structure
Potassium, a crucial element for life, boasts a fascinating electron configuration that dictates its chemical properties and reactivity. Understanding this configuration unlocks insights into its behavior in various chemical reactions and its role in biological systems. This comprehensive guide delves into the electron configuration of potassium, exploring its underlying principles, variations, and significance.
Understanding Electron Configuration
Before we delve into potassium's specific configuration, let's establish a foundational understanding of what electron configuration actually represents. An electron configuration describes the arrangement of electrons in the different energy levels (shells) and sublevels (subshells) within an atom. It follows specific rules governed by quantum mechanics, specifically the Aufbau principle, the Pauli exclusion principle, and Hund's rule.
-
Aufbau principle: Electrons fill the lowest energy levels first, progressing to higher energy levels as they are filled. This is analogous to building a structure from the ground up—you wouldn't start with the roof!
-
Pauli exclusion principle: Each orbital can hold a maximum of two electrons, each with opposite spins. This is like having two people comfortably fitting into a single seat, but no more.
-
Hund's rule: When filling orbitals of equal energy (degenerate orbitals), electrons first singly occupy each orbital before pairing up. This is like distributing students evenly across available desks before doubling up.
These principles govern the order in which electrons populate the atomic orbitals, resulting in a unique electron configuration for each element.
Potassium's Atomic Structure
Potassium (K) is an alkali metal with an atomic number of 19. This means a neutral potassium atom contains 19 protons in its nucleus and 19 electrons orbiting the nucleus. To determine its electron configuration, we systematically fill the electron shells and subshells according to the Aufbau principle, Pauli exclusion principle, and Hund's rule.
Determining Potassium's Electron Configuration
The electron configuration of potassium is typically represented in two common ways:
- Full electron configuration: This notation explicitly lists all occupied orbitals and subshells.
- Condensed electron configuration: This uses the noble gas notation as a shorthand, representing inner shell electrons with the symbol of the preceding noble gas.
Let's explore both:
1. Full Electron Configuration of Potassium
The full electron configuration of potassium is: 1s²2s²2p⁶3s²3p⁶4s¹
Let's break this down:
- 1s²: The first shell (n=1) contains one subshell (s), which holds a maximum of two electrons.
- 2s²: The second shell (n=2) also contains an 's' subshell, holding another two electrons.
- 2p⁶: The second shell also contains three 'p' subshells (px, py, pz), each holding two electrons, totaling six electrons.
- 3s²: The third shell (n=3) has an 's' subshell holding two electrons.
- 3p⁶: The third shell also has three 'p' subshells holding a total of six electrons.
- 4s¹: Finally, the fourth shell (n=4) has an 's' subshell with a single electron.
2. Condensed Electron Configuration of Potassium
The condensed electron configuration simplifies the notation by using the symbol of the preceding noble gas to represent the filled inner shells. The noble gas preceding potassium is Argon (Ar), which has the electron configuration 1s²2s²2p⁶3s²3p⁶. Therefore, the condensed electron configuration of potassium is: [Ar]4s¹
This notation is more concise and highlights the valence electron, which is crucial in determining potassium's chemical behavior.
Significance of Potassium's Electron Configuration
Potassium's electron configuration, particularly its single valence electron in the 4s orbital, is the key to understanding its chemical and biological properties.
1. Reactivity:
The single electron in the 4s orbital is relatively loosely held, making potassium highly reactive. It readily loses this electron to achieve a stable, filled outer shell configuration, resembling the noble gas Argon. This loss of an electron results in the formation of a K⁺ ion, a cation with a +1 charge. This tendency to lose an electron explains potassium's strong reducing properties and its vigorous reactions with water and other oxidizing agents.
2. Ionic Bonding:
Potassium's eagerness to lose its valence electron drives the formation of ionic bonds with electronegative elements like chlorine (Cl). In the reaction with chlorine, potassium loses its electron to form K⁺, while chlorine gains this electron to form Cl⁻. The electrostatic attraction between these oppositely charged ions results in the formation of potassium chloride (KCl), a common salt.
3. Biological Role:
Potassium's role in biological systems is inextricably linked to its electron configuration and reactivity. Potassium ions (K⁺) are essential for maintaining proper fluid balance, nerve impulse transmission, and muscle contraction. The ability of potassium ions to move across cell membranes is critical for these functions, making it a vital element for life. Its single valence electron facilitates the ease with which it forms ions and interacts with other biological molecules.
4. Spectroscopic Properties:
The electron configuration also influences potassium's spectroscopic properties. When potassium atoms absorb energy, their electrons can transition to higher energy levels. When these excited electrons return to their ground state, they emit light of characteristic wavelengths, leading to a distinctive spectrum. This spectral analysis can be used for potassium identification and quantification.
Variations and Exceptions
While the standard electron configuration we've discussed is the most common and stable, there can be slight variations under specific circumstances, such as:
- Excited States: If potassium atoms absorb sufficient energy, an electron can be promoted to a higher energy level. This creates an excited state with a different electron configuration, but it's generally short-lived and the atom quickly returns to its ground state.
- Ions: As mentioned, potassium readily loses its valence electron to form a K⁺ ion. The electron configuration of the K⁺ ion is [Ar], reflecting the loss of the 4s electron.
These variations, though less common, highlight the dynamic nature of electron configurations and the influence of external factors.
Conclusion
The electron configuration of potassium, 1s²2s²2p⁶3s²3p⁶4s¹ or [Ar]4s¹, is fundamental to understanding its properties and behavior. This simple configuration, with its single valence electron, dictates its high reactivity, its formation of ionic bonds, and its crucial biological role. The principles governing electron configurations provide a powerful framework for predicting the chemical and physical properties of elements, illustrating the elegant connection between atomic structure and macroscopic behavior. Understanding this configuration allows us to appreciate potassium's importance in both chemical reactions and life itself. Further exploration into the complexities of quantum mechanics can provide even deeper insights into the intricacies of atomic structure and its impact on the properties of matter.
Latest Posts
Latest Posts
-
Is Coal And Charcoal The Same
Apr 01, 2025
-
What Are The Common Multiples Of 3 And 5
Apr 01, 2025
-
Lcm Of 2 And 3 And 6
Apr 01, 2025
-
What Is The Prime Factorization 81
Apr 01, 2025
-
No Name This Compound According To Iupac Nomenclature Rules Responses
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about What Is Electron Configuration Of Potassium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
