Is Coal And Charcoal The Same
Juapaving
Apr 01, 2025 · 6 min read
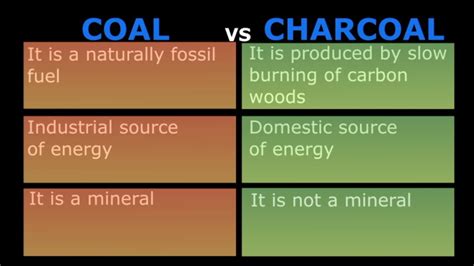
Table of Contents
Is Coal and Charcoal the Same? Understanding the Key Differences
Coal and charcoal might seem similar at first glance – both are black, both burn, and both have been used as fuel sources for centuries. However, a closer examination reveals significant differences in their origin, composition, properties, and uses. Understanding these distinctions is crucial, whether you're a curious student, a homeowner considering fuel options, or a professional in the energy sector. This comprehensive guide dives deep into the differences between coal and charcoal, clarifying common misconceptions and highlighting their unique characteristics.
The Origin Story: A Tale of Two Formations
The most fundamental difference between coal and charcoal lies in their origin. This divergence shapes their properties and ultimately, their applications.
Coal: A Geological Time Capsule
Coal is a fossil fuel, formed over millions of years from ancient plant matter buried under layers of sediment. The intense pressure and heat over geological timescales transformed this organic material, undergoing a process of carbonization. The resulting coal is primarily composed of carbon, but also contains varying amounts of other elements like hydrogen, oxygen, sulfur, and nitrogen, depending on the type of coal and the conditions during its formation. This process created different ranks of coal, each with distinct properties:
- Peat: The earliest stage, a brown, unconsolidated material still containing significant amounts of water.
- Lignite: A soft, brown coal with a higher carbon content than peat.
- Sub-bituminous coal: A more solid, dark brown coal with higher carbon content than lignite.
- Bituminous coal: A hard, black coal with a high carbon content, commonly used for power generation.
- Anthracite coal: The highest rank, a hard, black coal with the highest carbon content and the lowest moisture content.
Charcoal: A Product of Human Intervention
Charcoal, on the other hand, is a manufactured product. It's created through the pyrolysis of organic matter, typically wood. Pyrolysis is a thermal decomposition process that occurs in the absence of oxygen. Wood is heated in a controlled environment with limited oxygen supply, driving off volatile compounds like water, methanol, and acetic acid, leaving behind a porous, carbon-rich residue: charcoal. While the primary source is wood, other materials like bones, nutshells, and coconut shells can also be used to produce charcoal.
The process of charcoal production, while simple in principle, has evolved significantly over time. Traditional methods involve simple kilns or pits, while modern techniques employ sophisticated retort systems for greater efficiency and control over the process.
Compositional Contrast: A Chemical Comparison
The differences in formation lead to distinct chemical compositions. While both are primarily carbon, the exact composition and the presence of other elements significantly affect their properties.
Coal: A Complex Mixture
Coal's composition is complex and varies depending on its rank. The higher the rank, the greater the carbon content and the lower the volatile matter. The presence of sulfur, nitrogen, and other elements contributes to pollution when coal is burned, leading to environmental concerns. The calorific value, or the energy released upon combustion, also varies significantly across coal ranks, with anthracite having the highest.
Charcoal: Primarily Carbon, but not Pure
Charcoal is predominantly composed of carbon, typically around 80-90%, but also contains small amounts of other elements depending on the source material. The porosity of charcoal is a key feature, allowing for a large surface area, which is crucial for its various applications. Compared to coal, charcoal is generally considered cleaner-burning, producing fewer pollutants, although incomplete combustion can still release harmful substances.
Physical Properties: A Side-by-Side Look
The physical properties of coal and charcoal reflect their different origins and compositions. These differences are crucial in determining their uses.
Coal: Density and Hardness
Coal is a solid, relatively dense material. Its hardness varies with its rank, with anthracite being the hardest. Coal's density and hardness make it suitable for large-scale mining and transportation.
Charcoal: Porosity and Lightness
Charcoal is a porous, lightweight material. This porosity is a result of the pyrolysis process, creating a large surface area. This high surface area is responsible for its absorptive properties and its effectiveness as a fuel source. The lightness of charcoal makes it easier to handle and transport in smaller quantities.
Applications: Diverse Uses for Different Materials
The differing properties of coal and charcoal lead to significantly different applications.
Coal: Power Generation and Industry
Coal's primary use is in power generation, where it's burned to produce steam that drives turbines. It's also used in various industrial processes, including the production of steel, cement, and chemicals. However, due to its high carbon content and the pollutants released during combustion, the use of coal is facing increasing scrutiny.
Charcoal: Cooking, Art, and Filtration
Charcoal has a broader range of applications. Its most common use is as a fuel source for cooking, especially in barbecues and grills, appreciated for its ability to impart a distinct flavor to food. Its porous nature makes it useful as an absorbent, used in water filtration and for removing odors. Artists also utilize charcoal for drawing and sketching. Its porous structure also contributes to its use in industrial processes such as metallurgy.
Environmental Impact: A Crucial Consideration
Both coal and charcoal have environmental impacts, but the scale and nature of these impacts differ significantly.
Coal: A Major Contributor to Climate Change
Coal combustion is a major source of greenhouse gas emissions, contributing significantly to climate change. It also releases other pollutants like sulfur dioxide and nitrogen oxides, leading to acid rain and air pollution. These environmental concerns have led to a global push towards reducing coal consumption and transitioning to cleaner energy sources.
Charcoal: Sustainable Production and Use
While charcoal production can contribute to deforestation if not managed sustainably, the environmental impact is generally less significant than that of coal. The emissions during charcoal combustion are lower compared to coal. However, improper disposal and incomplete combustion can still contribute to air pollution.
Conclusion: A Clear Distinction
While both coal and charcoal are carbon-rich materials used as fuel sources, their origins, compositions, properties, and applications differ significantly. Coal, a fossil fuel formed over millions of years, is primarily used for large-scale power generation and industrial processes, but its use is increasingly challenged due to its significant environmental impact. Charcoal, a manufactured product, has a wider range of applications, from cooking to art and filtration, and while its production needs careful management, its environmental impact is comparatively lower. Understanding these crucial differences is vital for making informed choices about energy consumption and resource management in a world increasingly focused on sustainability.
Latest Posts
Latest Posts
-
How Many Miles Are In 10 Kilometers
Apr 02, 2025
-
Where In A Plant Cell Does Photosynthesis Occur
Apr 02, 2025
-
A Motor And A Generator Are
Apr 02, 2025
-
Allows Materials In And Out Of The Cell
Apr 02, 2025
-
Which Of The Following Is Matched Correctly
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Is Coal And Charcoal The Same . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
