No Name This Compound According To Iupac Nomenclature Rules. Responses
Juapaving
Apr 01, 2025 · 7 min read
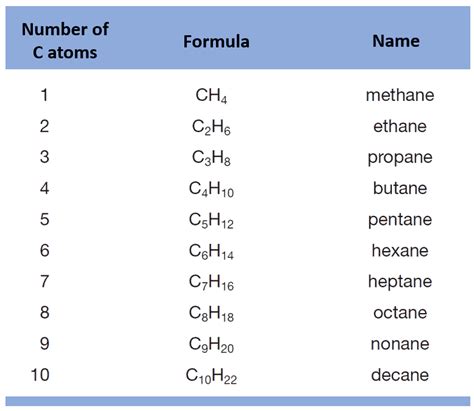
Table of Contents
Naming Organic Compounds: A Deep Dive into IUPAC Nomenclature
The International Union of Pure and Applied Chemistry (IUPAC) nomenclature is the globally accepted system for naming organic compounds. Its purpose is to ensure that every organic molecule has a unique and unambiguous name, preventing confusion and facilitating clear communication among chemists worldwide. This comprehensive guide will delve into the intricate rules of IUPAC nomenclature, providing a robust understanding of how to name various organic compounds, including those with complex structures. We’ll tackle alkanes, alkenes, alkynes, alkyl halides, alcohols, aldehydes, ketones, carboxylic acids, and more, offering numerous examples and explanations along the way.
Understanding the Fundamentals of IUPAC Nomenclature
Before delving into the complexities, let's establish the fundamental principles:
1. Identifying the Parent Chain: The Foundation of Naming
The first step in naming any organic compound is identifying the parent chain. This is the longest continuous carbon chain within the molecule. Even if the longest chain isn't in a straight line, it must be identified to form the basis of the name.
Example: Consider a molecule with a branched structure. You need to meticulously trace the carbons to find the longest continuous chain, regardless of the branching.
2. Numbering the Carbon Atoms: Establishing a Framework
Once the parent chain is identified, the next crucial step is numbering the carbon atoms. Numbering starts from the end of the chain that gives the substituents the lowest possible numbers. This ensures the name is as concise and unambiguous as possible.
Example: If a substituent is on carbon 2 and another is on carbon 4, the name will be shorter and clearer than if you numbered it with the substituents at positions 3 and 5.
3. Identifying and Naming Substituents: Adding Detail to the Structure
Substituents are atoms or groups of atoms attached to the parent chain. These are crucial components in determining the complete name of the molecule. Knowing the substituent's name is paramount. Common substituents include alkyl groups (methyl, ethyl, propyl, etc.), halogens (fluoro, chloro, bromo, iodo), and functional groups (hydroxyl, carboxyl, etc.).
Example: A methyl group (–CH₃) attached to the parent chain is named "methyl." A chlorine atom (–Cl) is named "chloro."
4. Combining Parent Chain and Substituents: Constructing the Full Name
The final step is combining the information from steps 1-3 to create the complete IUPAC name. The name follows a specific format:
- Number(s) of substituent(s) + Name of substituent(s) + Name of parent chain
Example: If a molecule has a parent chain of 5 carbons (pentane) with a methyl group on carbon 2, the name would be 2-methylpentane.
Naming Alkanes: The Simplest Hydrocarbons
Alkanes are saturated hydrocarbons containing only single bonds. Their names follow a straightforward pattern:
- Meth- (1 carbon)
- Eth- (2 carbons)
- Prop- (3 carbons)
- But- (4 carbons)
- Pent- (5 carbons)
- Hex- (6 carbons)
- Hept- (7 carbons)
- Oct- (8 carbons)
- Non- (9 carbons)
- Dec- (10 carbons)
And so on. To the root name, add "-ane" to indicate the alkane.
Examples:
- CH₄: Methane
- CH₃CH₃: Ethane
- CH₃CH₂CH₃: Propane
- CH₃CH₂CH₂CH₃: Butane
For branched alkanes, you'll need to identify the longest chain, number the carbons, name the substituents, and combine them as described earlier.
Branching Out: Dealing with Alkyl Substituents
Alkyl substituents are formed by removing a hydrogen atom from an alkane. These are named by replacing the "-ane" suffix with "-yl."
Examples:
- CH₃– (methyl)
- CH₃CH₂– (ethyl)
- CH₃CH₂CH₂– (propyl)
- CH₃CH₂CH₂CH₂– (butyl)
When multiple substituents are present, list them alphabetically, ignoring prefixes like di-, tri-, tetra-, etc., unless they are part of the substituent's name itself (e.g., isopropyl). Use hyphens to separate numbers and words. Use commas to separate numbers if multiple substituents are on the same carbon.
Example: A molecule with a six-carbon chain (hexane) with a methyl group on carbon 2 and an ethyl group on carbon 4 would be named 4-ethyl-2-methylhexane.
Delving into Unsaturated Hydrocarbons: Alkenes and Alkynes
Alkenes contain at least one carbon-carbon double bond, while alkynes contain at least one carbon-carbon triple bond. Naming these follows similar principles as alkanes, with a few crucial additions:
- Locate the double or triple bond: Number the carbon chain to give the double or triple bond the lowest possible number.
- Suffix change: Replace the "-ane" suffix with "-ene" for alkenes and "-yne" for alkynes.
Examples:
- CH₂=CH₂: Ethene
- CH₃CH=CH₂: Propene
- CH≡CH: Ethyne (also known as acetylene)
If multiple double or triple bonds are present, use prefixes like "di-" (two), "tri-" (three), etc., and indicate the location of each bond. The numbers are separated by commas.
Example: CH₂=CHCH=CH₂ is 1,3-butadiene.
Incorporating Functional Groups: Adding Complexity
Functional groups are specific groups of atoms within a molecule that confer characteristic chemical properties. These groups influence the naming process significantly. Each functional group has its own specific naming conventions.
Alcohols (-OH): The Hydroxyl Group
Alcohols contain a hydroxyl group (-OH). The naming convention involves:
- Identifying the parent chain: The longest chain containing the -OH group.
- Numbering the carbon chain: The -OH group gets the lowest possible number.
- Suffix change: Replace the "-e" of the alkane with "-ol."
Example: CH₃CH₂OH is ethanol.
Aldehydes (-CHO): The Carbonyl Group at the End
Aldehydes have a carbonyl group (-CHO) at the end of the carbon chain. The naming convention uses:
- Identifying the parent chain: The longest chain containing the -CHO group.
- Suffix change: Replace the "-e" of the alkane with "-al." The carbonyl carbon is always carbon 1.
Example: CH₃CHO is ethanal.
Ketones (R-CO-R'): The Carbonyl Group in the Middle
Ketones have a carbonyl group (-CO-) within the carbon chain. The naming convention involves:
- Identifying the parent chain: The longest chain containing the carbonyl group.
- Numbering the carbon chain: The carbonyl group gets the lowest possible number.
- Suffix change: Replace the "-e" of the alkane with "-one."
Example: CH₃COCH₃ is propanone (also known as acetone).
Carboxylic Acids (-COOH): The Carboxyl Group
Carboxylic acids possess a carboxyl group (-COOH). The naming convention:
- Identifying the parent chain: The longest chain containing the -COOH group.
- Suffix change: Replace the "-e" of the alkane with "-oic acid." The carboxyl carbon is always carbon 1.
Example: CH₃COOH is ethanoic acid (also known as acetic acid).
Halides: Incorporating Halogens
Halogens (fluorine, chlorine, bromine, iodine) are treated as substituents. Their names are fluoro-, chloro-, bromo-, and iodo-, respectively. They are listed alphabetically with other substituents.
Example: CH₃CHClCH₃ is 2-chloropropane.
Dealing with Complex Structures: A Multifaceted Approach
Many organic molecules possess complex structures with multiple functional groups and substituents. In such cases, a hierarchical order of precedence is applied. This order ensures that the most significant functional group determines the base name and the other groups are treated as substituents.
Advanced Nomenclature Considerations
This introduction provides a solid foundation in IUPAC nomenclature. However, many more nuanced rules exist to handle stereoisomers (cis/trans, E/Z), cyclic compounds, aromatic compounds, and other complexities. These require dedicated study and practice. Consult comprehensive organic chemistry textbooks or specialized nomenclature guides for a thorough understanding of these advanced aspects.
Conclusion: Mastering the Art of Naming Organic Compounds
Mastering IUPAC nomenclature requires careful attention to detail, practice, and a systematic approach. By understanding the fundamental principles – identifying the parent chain, numbering carbons, naming substituents, and incorporating functional groups – you can confidently name a vast array of organic compounds. While the rules may seem initially complex, the system's elegance lies in its unambiguous and universally accepted approach to naming organic molecules, ensuring clear communication in the field of chemistry. Consistent practice and consulting relevant resources will solidify your understanding and proficiency in this crucial aspect of organic chemistry.
Latest Posts
Latest Posts
-
A Letter Or Symbol That Represents A Missing Value
Apr 02, 2025
-
A Slender Homogeneous Rod Of Length 2l
Apr 02, 2025
-
Cervical Thoracic And Lumbar Vertebrae Differences
Apr 02, 2025
-
Are All Cells The Same Shape And Size
Apr 02, 2025
-
What Are Rows Called In The Periodic Table
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about No Name This Compound According To Iupac Nomenclature Rules. Responses . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
