What Information Does The Electronic Configuration Of An Atom Provide
Juapaving
Mar 23, 2025 · 6 min read
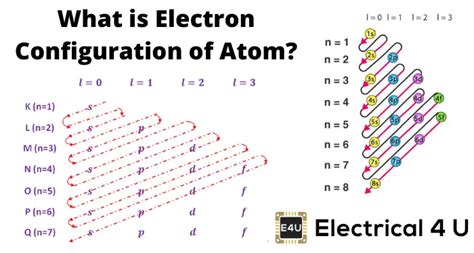
Table of Contents
What Information Does the Electronic Configuration of an Atom Provide?
The electronic configuration of an atom provides a fundamental blueprint of its properties and behavior. It dictates how an atom will interact with other atoms, forming molecules and influencing the macroscopic properties of matter. Understanding electronic configuration is crucial in chemistry, physics, and materials science. This comprehensive guide delves into the wealth of information encoded within an atom's electron arrangement.
Understanding Electronic Configuration: A Foundation
An atom's electronic configuration describes the arrangement of electrons in its various energy levels and sublevels. It's a shorthand notation representing the distribution of electrons within the atom's orbitals. This arrangement is governed by the principles of quantum mechanics, specifically the Pauli Exclusion Principle and Hund's Rule, which dictate how electrons fill orbitals to achieve the lowest possible energy state.
Key Concepts:
- Energy Levels (Shells): Electrons reside in distinct energy levels, denoted by principal quantum numbers (n = 1, 2, 3...). Levels closer to the nucleus have lower energy.
- Sublevels (Subshells): Each energy level contains sublevels, designated by letters (s, p, d, f). These sublevels have slightly different energies within a given shell.
- Orbitals: Each sublevel comprises one or more orbitals, which are regions of space where an electron is most likely to be found. 's' sublevel has one orbital, 'p' has three, 'd' has five, and 'f' has seven.
- Electrons: Each orbital can hold a maximum of two electrons, with opposite spins (Pauli Exclusion Principle).
Information Revealed by Electronic Configuration:
The electronic configuration provides a wealth of information, impacting various aspects of an atom's characteristics. Let's explore these aspects in detail:
1. Chemical Properties and Reactivity:
The valence electrons, the outermost electrons in the highest energy level, are primarily responsible for an atom's chemical behavior. The electronic configuration directly reveals the number of valence electrons. This number determines an atom's tendency to gain, lose, or share electrons to achieve a stable electron configuration (usually a full outermost shell, often resembling a noble gas).
- Determining Valency: Knowing the number of valence electrons allows us to predict the valency of an element – its combining capacity with other atoms. For example, oxygen (electronic configuration 1s²2s²2p⁴) has six valence electrons and a valency of 2, often forming two bonds to complete its octet.
- Predicting Bond Formation: Electronic configuration helps predict the type of bonds an atom will form (ionic, covalent, metallic). Atoms with low ionization energies (easily losing electrons) tend to form ionic bonds with atoms having high electron affinities (easily gaining electrons). Atoms with similar electronegativities often form covalent bonds by sharing electrons.
- Understanding Oxidation States: The electronic configuration helps determine the possible oxidation states of an element—the apparent charge an atom has in a compound due to electron transfer or sharing.
2. Atomic Radius and Shielding Effect:
The electronic configuration influences the size of an atom. As you move down a group in the periodic table, the number of shells increases, leading to a larger atomic radius. Within a period, increasing nuclear charge pulls electrons closer, slightly decreasing atomic radius.
The shielding effect—the reduction of the effective nuclear charge on valence electrons due to inner electrons—is also related to electronic configuration. Inner electrons partially shield valence electrons from the full positive charge of the nucleus. This shielding effect influences the ionization energy and electron affinity of an atom.
3. Ionization Energy and Electron Affinity:
- Ionization Energy: The energy required to remove an electron from an atom is called ionization energy. The electronic configuration helps predict ionization energy trends. Elements with a full or half-filled outermost shell have higher ionization energies because removing an electron disrupts the stable configuration.
- Electron Affinity: The energy change when an atom gains an electron is electron affinity. Electronic configuration influences this. Atoms with nearly full outer shells (e.g., halogens) have high electron affinities because gaining an electron leads to a stable configuration.
4. Magnetic Properties:
The electronic configuration determines the magnetic properties of an atom or ion. If an atom has unpaired electrons in its orbitals, it will be paramagnetic, meaning it will be attracted to a magnetic field. If all electrons are paired, the atom will be diamagnetic, meaning it will be slightly repelled by a magnetic field. Hund's rule, which states that electrons will individually occupy orbitals within a subshell before pairing up, is crucial in determining the presence of unpaired electrons.
5. Metallic Character:
The electronic configuration strongly influences an element's metallic character. Elements with few valence electrons and low ionization energies tend to exhibit metallic properties like conductivity and malleability. This is because their valence electrons are loosely held and can move freely throughout the metallic lattice, facilitating electrical and thermal conductivity.
6. Spectral Lines and Atomic Emission Spectra:
When electrons transition between energy levels, they absorb or emit photons of specific energies. These energy differences correspond to specific wavelengths of light, creating characteristic spectral lines. The electronic configuration dictates the possible energy transitions and thus the wavelengths observed in an element's atomic emission spectrum. This spectrum acts as a unique fingerprint for each element.
7. Periodicity and the Periodic Table:
The periodic table is organized based on the electronic configurations of elements. Elements in the same group have similar valence electron configurations, leading to similar chemical properties. The periodic trends in atomic radius, ionization energy, and electron affinity are directly related to the systematic changes in electronic configuration across the periodic table. Understanding electronic configuration clarifies the rationale behind the periodic table's structure and the recurring trends in properties.
8. Predicting the Properties of Unknown Elements:
Electronic configuration allows scientists to predict the properties of yet-undiscovered elements. By extrapolating trends based on known elements, we can anticipate their chemical behavior, atomic radius, and other characteristics. This predictive power is essential in the exploration and understanding of the periodic table's furthest reaches.
Advanced Concepts and Applications:
The information derived from electronic configuration extends beyond basic chemical properties. More sophisticated applications include:
- Molecular Orbital Theory: This theory utilizes electronic configuration to describe bonding in molecules by considering the combination of atomic orbitals to form molecular orbitals.
- Band Theory of Solids: This theory explains the electrical conductivity of solids based on the arrangement of energy levels in a crystal lattice, directly related to the electronic configurations of constituent atoms.
- Spectroscopy and Analytical Chemistry: Spectroscopic techniques like UV-Vis, IR, and NMR spectroscopy rely on understanding electronic configurations to interpret the interaction of molecules with electromagnetic radiation.
- Materials Science and Engineering: The design and synthesis of novel materials with specific properties (e.g., superconductivity, magnetism) are guided by considerations of electronic configuration.
Conclusion:
The electronic configuration of an atom is a cornerstone of chemistry and related fields. It encodes a wealth of information, providing insights into an atom's chemical reactivity, size, magnetic properties, spectral behavior, and more. By understanding electronic configuration, we gain a profound comprehension of the properties of matter and the underlying principles governing chemical interactions, laying the foundation for the exploration of a vast range of scientific and technological advancements. From predicting the behavior of known elements to designing novel materials, the power and versatility of electronic configuration remain essential in driving scientific progress.
Latest Posts
Latest Posts
-
Are All Bronsted Acids Lewis Acids
Mar 25, 2025
-
Mean Value Theorem Vs Intermediate Value Theorem
Mar 25, 2025
-
The Greater The Concentration Gradient The Faster The Diffusion Rate
Mar 25, 2025
-
Select The Components Of A Nucleotide
Mar 25, 2025
-
Which Of The Following Statements About Vaccines Is True
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about What Information Does The Electronic Configuration Of An Atom Provide . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
