Select The Components Of A Nucleotide
Juapaving
Mar 25, 2025 · 6 min read
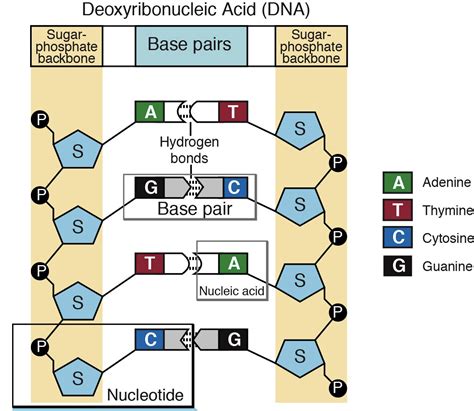
Table of Contents
Selecting the Components of a Nucleotide: A Deep Dive into the Building Blocks of Life
Nucleotides are the fundamental building blocks of nucleic acids, DNA and RNA, the molecules that carry genetic information in all living organisms. Understanding the components of a nucleotide is crucial to comprehending the intricacies of genetics, molecular biology, and the very essence of life itself. This comprehensive guide will delve into the specific components, their chemical structures, and the roles they play in the overall functionality of nucleotides and nucleic acids.
The Tripartite Nature of Nucleotides
A nucleotide is comprised of three essential components:
- A nitrogenous base: This is a heterocyclic aromatic ring compound containing nitrogen atoms. These bases are crucial for encoding genetic information.
- A pentose sugar: This is a five-carbon sugar molecule, either ribose (in RNA) or deoxyribose (in DNA), that forms the backbone of the nucleic acid strand.
- A phosphate group: This is a negatively charged group consisting of a phosphorus atom bonded to four oxygen atoms, providing the structural backbone and crucial chemical properties.
Let's examine each component in detail:
1. Nitrogenous Bases: The Alphabet of Life
Nitrogenous bases are categorized into two main groups based on their chemical structure:
1.1 Purines: Double-Ringed Structures
Purines are characterized by a double-ring structure, consisting of a six-membered ring fused to a five-membered ring. The two principal purine bases found in DNA and RNA are:
-
Adenine (A): Adenine is a crucial base paired with Thymine (in DNA) and Uracil (in RNA). Its structure includes an amino group (-NH2) at the 6 position. This amino group plays a vital role in hydrogen bonding, enabling base pairing.
-
Guanine (G): Guanine is another crucial purine base, pairing with Cytosine in both DNA and RNA. It features an oxygen atom (=O) and an amino group (-NH2) at specific positions on its structure. The precise arrangement of these groups is fundamental for the specific hydrogen bonding with cytosine.
1.2 Pyrimidines: Single-Ringed Structures
Pyrimidines have a single six-membered ring structure. The pyrimidine bases found in DNA and RNA include:
-
Cytosine (C): Cytosine pairs with Guanine in both DNA and RNA. It possesses an amino group (-NH2) and a carbonyl group (=O). The arrangement of these groups enables its specific hydrogen bonding with guanine.
-
Thymine (T): Thymine is found exclusively in DNA, pairing with Adenine. It contains two carbonyl groups (=O) and a methyl group (-CH3). The methyl group distinguishes thymine from uracil and is crucial for DNA stability.
-
Uracil (U): Uracil replaces thymine in RNA, also pairing with Adenine. It lacks the methyl group found in thymine and has two carbonyl groups (=O). The absence of the methyl group makes uracil slightly less stable than thymine, which is appropriate for the more transient nature of RNA.
2. Pentose Sugars: The Backbone of Nucleic Acids
The pentose sugar forms the backbone of the nucleotide, linking the nitrogenous base to the phosphate group. Two types of pentose sugars are found in nucleic acids:
2.1 Ribose: The Sugar of RNA
Ribose is a five-carbon sugar with a hydroxyl (-OH) group attached to the 2' carbon atom. This hydroxyl group is crucial for the chemical reactivity of RNA, making it less stable but more versatile than DNA. The presence of the 2'-OH group allows for RNA's ability to fold into complex three-dimensional structures.
2.2 Deoxyribose: The Sugar of DNA
Deoxyribose is a derivative of ribose, differing only in the absence of a hydroxyl (-OH) group at the 2' carbon atom. Instead, it has a hydrogen atom (-H) at this position. This lack of a hydroxyl group makes DNA more stable and less prone to hydrolysis than RNA. This stability is crucial for the long-term storage of genetic information.
3. Phosphate Group: The Linking Agent and Charge Carrier
The phosphate group is the negatively charged component of a nucleotide, typically bonded to the 5' carbon atom of the pentose sugar. It carries a negative charge at physiological pH, making nucleotides acidic molecules. This negative charge is crucial for the overall structure and function of nucleic acids.
The phosphate group acts as a linking agent, connecting the 5' carbon of one nucleotide to the 3' carbon of the next nucleotide, forming the phosphodiester bond that constitutes the backbone of the nucleic acid chain. This linkage is essential for creating the linear polymer structure of DNA and RNA.
The Nucleotide's Role in Nucleic Acid Formation
Once the three components—nitrogenous base, pentose sugar, and phosphate group—are assembled, a nucleoside is formed. When a phosphate group is added to a nucleoside, a nucleotide is created. Multiple nucleotides link together through phosphodiester bonds to create the polynucleotide chains that form DNA and RNA.
The sequence of nitrogenous bases along the polynucleotide chain determines the genetic information encoded in the molecule. The precise pairing of bases (A with T or U, and G with C) through hydrogen bonds is essential for the double-helical structure of DNA and the diverse secondary structures of RNA.
Beyond the Basics: Modified Nucleotides and Their Significance
While the basic components described above are common to all nucleotides, modifications can occur, significantly impacting the function of nucleic acids. These modifications include:
- Methylation: The addition of a methyl group (-CH3) to a base, often influencing gene expression.
- Acetylation: The addition of an acetyl group (-COCH3), affecting DNA stability and chromatin structure.
- Other modifications: Various other chemical modifications can occur, impacting the stability, interactions, and overall functions of nucleic acids.
Clinical Significance and Further Research
Understanding nucleotide components and their modifications is crucial for various fields:
- Medical research: Mutations in DNA, often involving changes in nucleotides, are associated with numerous genetic diseases.
- Drug development: Many drugs target nucleotide metabolism or nucleic acid structure, offering treatments for various diseases like viral infections and cancer.
- Biotechnology: Manipulating nucleotides is essential for various biotechnology applications, including genetic engineering and gene therapy.
Further research continues to unveil the intricacies of nucleotide structure, function, and modifications, revealing new insights into the fundamental processes of life and providing new avenues for therapeutic interventions.
Conclusion: A Foundation for Life
The components of a nucleotide—the nitrogenous base, pentose sugar, and phosphate group—are not merely individual molecules; they are the intricate puzzle pieces that assemble to form the foundation of life itself. Their precise chemical structures, interactions, and modifications dictate the information encoded in DNA and RNA, influencing gene expression, protein synthesis, and a myriad of cellular processes. A deep understanding of these fundamental building blocks is essential for advancing our knowledge of molecular biology, genetics, and the continuing quest to unravel the complexities of life.
Latest Posts
Latest Posts
-
Why Is Blood Considered To Be A Connective Tissue
May 09, 2025
-
Name For A Group Of Penguins
May 09, 2025
-
What Is 1 Billion X 1 Trillion
May 09, 2025
-
Vinegar Is An Acid Or Base
May 09, 2025
-
What Is 3 Out Of 4 As A Percentage
May 09, 2025
Related Post
Thank you for visiting our website which covers about Select The Components Of A Nucleotide . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.