Period 3 Contains A Total Of Elements
Juapaving
Mar 26, 2025 · 6 min read
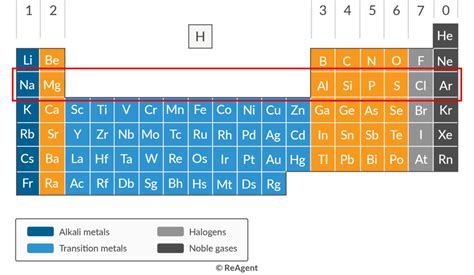
Table of Contents
Period 3: A Deep Dive into the Elements and Their Properties
Period 3 of the periodic table, encompassing elements from sodium (Na) to argon (Ar), represents a fascinating chapter in the study of chemistry. This period showcases a beautiful transition in properties, highlighting the influence of increasing nuclear charge and the filling of the 3p orbitals. This article will delve deep into the characteristics of each element, exploring their physical and chemical properties, applications, and the underlying reasons for their behavior. We’ll examine their electronic configurations, atomic radii, ionization energies, electronegativity, and oxidation states, all crucial factors in determining their reactivity and applications.
Understanding Period 3 Trends
Before we explore each element individually, let's establish the fundamental trends observed across Period 3. These trends are crucial for understanding the chemical behavior of these elements:
1. Atomic Radius:
The atomic radius generally decreases across Period 3 from left to right. This is because, while additional electrons are added to the same principal energy level (n=3), the nuclear charge (number of protons) increases. The increased positive charge pulls the electrons closer to the nucleus, resulting in a smaller atomic radius.
2. Ionization Energy:
Ionization energy, the energy required to remove an electron from an atom, generally increases across Period 3. The stronger nuclear attraction in elements towards the right side of the period makes it more difficult to remove an electron.
3. Electronegativity:
Electronegativity, the ability of an atom to attract electrons in a chemical bond, also generally increases across Period 3. This follows the same reasoning as ionization energy – a stronger nuclear charge attracts shared electrons more effectively.
4. Metallic Character:
Metallic character generally decreases across Period 3. This is directly related to the increase in ionization energy and electronegativity. Elements on the left are more metallic (easily lose electrons), while those on the right are less metallic (less likely to lose electrons).
Exploring the Elements of Period 3
Now, let's examine each element in Period 3 individually:
1. Sodium (Na):
- Electronic Configuration: [Ne] 3s¹
- Properties: Sodium is a soft, silvery-white, highly reactive alkali metal. It readily loses its single valence electron to achieve a stable noble gas configuration, exhibiting a +1 oxidation state. It reacts vigorously with water, producing hydrogen gas and sodium hydroxide.
- Applications: Sodium is used in various applications, including the production of sodium compounds (like sodium hydroxide and sodium carbonate), in sodium-vapor lamps, and as a heat transfer medium in some industrial processes.
2. Magnesium (Mg):
- Electronic Configuration: [Ne] 3s²
- Properties: Magnesium is a relatively reactive alkaline earth metal. It loses two valence electrons to achieve a stable configuration, exhibiting a +2 oxidation state. It burns brightly in air, producing a dazzling white light. It is lighter than aluminum, but stronger than many metals.
- Applications: Magnesium is extensively used in lightweight alloys for aerospace applications, in the production of various metal products and as a reducing agent in metallurgy. It's also a crucial element in chlorophyll, essential for photosynthesis in plants.
3. Aluminium (Al):
- Electronic Configuration: [Ne] 3s²3p¹
- Properties: Aluminium is a lightweight, silvery-white metal with high electrical and thermal conductivity. It's amphoteric, meaning it can react with both acids and bases. It forms a protective oxide layer that prevents further corrosion.
- Applications: Aluminum is ubiquitous; its applications include packaging, transportation (cars, airplanes), construction, electrical wiring, and kitchenware. Its lightness, strength, and corrosion resistance make it highly versatile.
4. Silicon (Si):
- Electronic Configuration: [Ne] 3s²3p²
- Properties: Silicon is a metalloid, exhibiting properties of both metals and nonmetals. It’s a semiconductor, meaning its electrical conductivity can be controlled. It exists primarily as silica (SiO₂) in the earth's crust.
- Applications: Silicon is crucial in the semiconductor industry, forming the basis of microchips and integrated circuits. It’s also used in glass, ceramics, and various silicone-based polymers.
5. Phosphorus (P):
- Electronic Configuration: [Ne] 3s²3p³
- Properties: Phosphorus exists in several allotropic forms (different structural modifications), with white phosphorus being highly reactive and toxic, while red phosphorus is less reactive. It typically exhibits oxidation states of -3, +3, and +5.
- Applications: Phosphorus is an essential nutrient for plants and animals, primarily found in fertilizers. It's also used in matches, pesticides, and certain detergents.
6. Sulfur (S):
- Electronic Configuration: [Ne] 3s²3p⁴
- Properties: Sulfur is a nonmetal, existing in various allotropic forms, the most common being yellow rhombic sulfur. It’s relatively unreactive at room temperature but reacts with many elements when heated. It commonly exhibits oxidation states of -2, +4, and +6.
- Applications: Sulfur is widely used in the production of sulfuric acid (a crucial industrial chemical), in vulcanizing rubber, in fungicides, and in the production of various other chemicals.
7. Chlorine (Cl):
- Electronic Configuration: [Ne] 3s²3p⁵
- Properties: Chlorine is a highly reactive, toxic, greenish-yellow gas. It readily gains one electron to achieve a stable octet, exhibiting a -1 oxidation state. It's a powerful oxidizing agent.
- Applications: Chlorine is used extensively in water purification to disinfect and kill bacteria and viruses. It's also used in the production of various chemicals, including PVC plastics, and as a bleaching agent.
8. Argon (Ar):
- Electronic Configuration: [Ne] 3s²3p⁶
- Properties: Argon is a noble gas, meaning it's chemically inert and unreactive due to its full valence electron shell. It’s colorless and odorless.
- Applications: Argon is used as an inert atmosphere in welding and other industrial processes where reactivity needs to be avoided. It’s also used in incandescent and fluorescent lighting.
Period 3: A Summary of Interconnected Properties
The properties of Period 3 elements are intrinsically linked. The gradual increase in nuclear charge across the period leads to a systematic decrease in atomic radius, and increases in ionization energy and electronegativity. This directly impacts their chemical reactivity and the types of bonds they form. The transition from metallic to non-metallic character is a key observation, with sodium displaying strong metallic behavior and argon demonstrating complete chemical inertness.
Understanding these trends and the individual properties of each element provides a fundamental basis for comprehending the chemical reactions and applications involving Period 3 elements. These elements play crucial roles in various aspects of our daily lives, from the production of essential materials to sophisticated technological applications. Further study into their compounds and reactions reveals a wealth of additional insights into their behavior and importance. The systematic changes in their properties across the period provide a clear illustration of the periodic law and its power in predicting chemical behavior. Each element holds its unique place in the grand scheme of chemical interactions, and a detailed understanding of their properties is key to unlocking many areas of chemical and technological advancements.
Latest Posts
Latest Posts
-
How Many Electrons In Oxygen Atom
Mar 29, 2025
-
Whats The Prime Factorization Of 15
Mar 29, 2025
-
37 Inches Is How Many Feet
Mar 29, 2025
-
Find The Lcm Of 3 And 5
Mar 29, 2025
-
How Do You Do Average In Math
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Period 3 Contains A Total Of Elements . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
