How Many Electrons In Oxygen Atom
Juapaving
Mar 29, 2025 · 5 min read
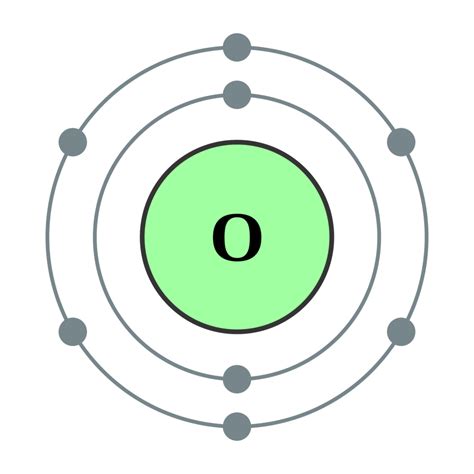
Table of Contents
How Many Electrons in an Oxygen Atom? A Deep Dive into Atomic Structure
Understanding the number of electrons in an oxygen atom is fundamental to grasping the basics of chemistry and atomic structure. This seemingly simple question opens the door to exploring a fascinating world of subatomic particles, electron shells, and the periodic table. This comprehensive guide will delve into the answer, explaining the underlying principles and exploring related concepts.
The Simple Answer: 8 Electrons
The oxygen atom, denoted by the symbol O, has eight electrons. This is a crucial piece of information that dictates its chemical properties and behavior. But simply stating the number doesn't fully explain the 'why' behind it. To truly understand, we need to explore the atom's structure.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
An atom is the fundamental building block of matter. It consists of three primary subatomic particles:
- Protons: Positively charged particles found in the atom's nucleus (center). The number of protons defines the element. Oxygen always has 8 protons.
- Neutrons: Neutrally charged particles also located in the nucleus. The number of neutrons can vary within an element, leading to isotopes. Oxygen has several isotopes, with the most common having 8 neutrons.
- Electrons: Negatively charged particles that orbit the nucleus in electron shells or energy levels. These electrons are responsible for chemical bonding and the atom's reactivity. The number of electrons in a neutral atom is equal to the number of protons.
The Role of Electron Shells and Energy Levels
Electrons don't just randomly orbit the nucleus; they exist in specific energy levels or shells. These shells are designated by numbers (1, 2, 3, and so on), with shell 1 being closest to the nucleus and having the lowest energy. Each shell can hold a maximum number of electrons:
- Shell 1: Can hold a maximum of 2 electrons.
- Shell 2: Can hold a maximum of 8 electrons.
- Shell 3: Can hold a maximum of 18 electrons.
- Higher shells: Can hold even more electrons.
The electron configuration of an atom describes how its electrons are distributed among these shells. For oxygen, with its 8 electrons, the configuration is:
- Shell 1: 2 electrons
- Shell 2: 6 electrons
This configuration is crucial in determining oxygen's chemical behavior. The outermost shell, called the valence shell (shell 2 in this case), contains the valence electrons. These are the electrons involved in chemical bonding. Oxygen has 6 valence electrons, making it highly reactive. It tends to gain two electrons to achieve a stable octet (8 electrons) in its valence shell, a configuration similar to the noble gas neon.
Isotopes of Oxygen: Variations in Neutron Count
While the number of protons and electrons defines an element, the number of neutrons can vary. These variations are called isotopes. Oxygen has three main stable isotopes:
- Oxygen-16 (¹⁶O): 8 protons, 8 neutrons, 8 electrons – This is the most abundant isotope.
- Oxygen-17 (¹⁷O): 8 protons, 9 neutrons, 8 electrons
- Oxygen-18 (¹⁸O): 8 protons, 10 neutrons, 8 electrons
Note that despite the different neutron counts, the number of electrons remains the same (8) in all isotopes of oxygen. The difference in neutron number affects the atomic mass but not the chemical properties significantly.
Oxygen's Chemical Behavior and Electron Configuration
Oxygen's six valence electrons make it highly reactive. It readily forms covalent bonds with other atoms to achieve a stable octet configuration. This explains its crucial role in many chemical processes, including:
- Respiration: Oxygen is vital for aerobic respiration, the process by which living organisms convert food into energy. This process involves the transfer of electrons in a series of redox reactions.
- Combustion: Oxygen supports combustion, the rapid oxidation of a substance, producing heat and light.
- Water Formation: Oxygen readily forms covalent bonds with two hydrogen atoms to create water (H₂O), a fundamental molecule for life.
The Periodic Table and Oxygen's Position
The periodic table is a powerful tool that organizes elements based on their atomic structure and properties. Oxygen's position in the table reflects its characteristics. It's located in Group 16 (also known as the chalcogens) and Period 2. This placement indicates its six valence electrons and its relatively small atomic size.
The elements within the same group share similar chemical properties due to having the same number of valence electrons. All chalcogens tend to gain two electrons to achieve a stable octet.
Beyond the Basics: Quantum Mechanics and Electron Orbitals
The simple shell model provides a good introductory understanding, but a more accurate description involves quantum mechanics and the concept of electron orbitals. Orbitals are regions within an atom where there's a high probability of finding an electron. These orbitals have specific shapes and energy levels, described by quantum numbers.
Oxygen's electron configuration in terms of orbitals is: 1s² 2s² 2p⁴. This notation specifies the principal quantum number (n), the azimuthal quantum number (l), and the magnetic quantum number (ml), providing a more precise description of electron location and energy.
Conclusion: The Significance of Oxygen's Eight Electrons
The seemingly straightforward question – "How many electrons in an oxygen atom?" – opens up a wide-ranging exploration of atomic structure, chemical bonding, and the periodic table. The eight electrons of oxygen, specifically its six valence electrons, are directly responsible for its high reactivity, its role in vital biological processes like respiration, and its ability to form crucial compounds like water. Understanding this fundamental aspect of oxygen's atomic structure is essential for grasping many fundamental concepts in chemistry and beyond. From simple shell models to the complexities of quantum mechanics, the journey into the world of the oxygen atom highlights the intricate beauty and precision of the natural world. Further research into electron behavior, bonding mechanisms, and the periodic table's organization will deepen your understanding of this essential element and its critical contributions to our world.
Latest Posts
Latest Posts
-
What Is 3 33333 As A Fraction
Apr 01, 2025
-
What Does A Prokaryotic Cell Not Have
Apr 01, 2025
-
What Are More Things About The Major Components Of Soil
Apr 01, 2025
-
What Is The Lcm Of 5 6 7
Apr 01, 2025
-
Find The Complementary And Supplementary Angles
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons In Oxygen Atom . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
