Nh4 3po4 Pb No3 4 Pb3 Po4 4 Nh4no3
Juapaving
Mar 27, 2025 · 5 min read
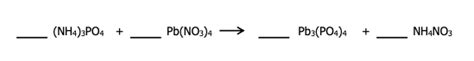
Table of Contents
Exploring the Reaction Between Ammonium Phosphate and Lead Nitrate: A Comprehensive Analysis of NH₄₃PO₄, Pb(NO₃)₄, Pb₃(PO₄)₄, and NH₄NO₃
The reaction between ammonium phosphate ((NH₄)₃PO₄) and lead(IV) nitrate (Pb(NO₃)₄) is a fascinating example of a double displacement reaction, resulting in the formation of lead(IV) phosphate (Pb₃(PO₄)₄) and ammonium nitrate (NH₄NO₃). This seemingly simple reaction presents a wealth of opportunities for exploring stoichiometry, solubility rules, and the properties of different ionic compounds. This article will delve into the details of this reaction, exploring its stoichiometry, the properties of the reactants and products, potential applications, and safety considerations.
Understanding the Reactants: (NH₄)₃PO₄ and Pb(NO₃)₄
Before diving into the reaction itself, let's examine the individual reactants: ammonium phosphate and lead(IV) nitrate.
Ammonium Phosphate ((NH₄)₃PO₄)
Ammonium phosphate is a white, crystalline salt that is highly soluble in water. It's commonly used as a fertilizer due to its high nitrogen and phosphorus content, crucial nutrients for plant growth. Its solubility stems from the strong electrostatic forces between the ammonium (NH₄⁺) cation and the phosphate (PO₄³⁻) anion. The ammonium ion acts as a weak acid, while the phosphate ion is a weak base. This amphoteric nature contributes to its versatility in various applications.
Key Properties of (NH₄)₃PO₄:
- Molar Mass: Approximately 149.09 g/mol
- Solubility: Highly soluble in water
- Appearance: White crystalline solid
- Applications: Fertilizer, flame retardant, yeast nutrient
Lead(IV) Nitrate (Pb(NO₃)₄)
Lead(IV) nitrate is a less common compound compared to lead(II) nitrate. It's a strong oxidizing agent and exists as a colorless to pale yellow crystalline solid. It's less stable than lead(II) nitrate and tends to decompose more readily. Its solubility in water is moderate, though less than that of ammonium phosphate. The high oxidation state of lead (+4) contributes to its oxidizing properties.
Key Properties of Pb(NO₃)₄:
- Molar Mass: Approximately 455.21 g/mol
- Solubility: Moderately soluble in water
- Appearance: Colorless to pale yellow crystals
- Applications: Relatively limited due to instability and toxicity. May find niche applications in specialized chemical processes.
The Reaction: (NH₄)₃PO₄ + Pb(NO₃)₄ → Pb₃(PO₄)₄ + NH₄NO₃
The reaction between ammonium phosphate and lead(IV) nitrate is a double displacement reaction, also known as a metathesis reaction. In this type of reaction, the cations and anions of two different ionic compounds switch places, forming two new compounds. The balanced chemical equation is:
3(NH₄)₃PO₄(aq) + 4Pb(NO₃)₄(aq) → Pb₃(PO₄)₄(s) + 12NH₄NO₃(aq)
This equation indicates that three moles of ammonium phosphate react with four moles of lead(IV) nitrate to produce one mole of lead(IV) phosphate and twelve moles of ammonium nitrate. The (aq) designation indicates that the substance is aqueous (dissolved in water), while (s) indicates a solid precipitate.
Understanding the Formation of the Precipitate: Pb₃(PO₄)₄
The driving force behind this reaction is the formation of lead(IV) phosphate, an insoluble salt. According to solubility rules, phosphates are generally insoluble except when combined with alkali metals (Group 1) and ammonium. Since lead(IV) is not an alkali metal or ammonium, lead(IV) phosphate precipitates out of the solution as a solid. This precipitation shifts the equilibrium to the right, favoring the product formation.
Understanding the Products: Pb₃(PO₄)₄ and NH₄NO₃
Let's examine the properties of the products formed in the reaction:
Lead(IV) Phosphate (Pb₃(PO₄)₄)
Lead(IV) phosphate is a solid precipitate formed during the reaction. It's insoluble in water and exhibits a unique crystalline structure. Its color varies depending on the purity and particle size. Due to the presence of lead, it is considered toxic and requires careful handling.
Key Properties of Pb₃(PO₄)₄:
- Molar Mass: Approximately 1168.23 g/mol
- Solubility: Insoluble in water
- Appearance: Typically a white solid, but can vary in appearance.
- Toxicity: Highly toxic due to the presence of lead.
Ammonium Nitrate (NH₄NO₃)
Ammonium nitrate is a highly soluble salt that remains in solution after the reaction. It's a common component of fertilizers and explosives. Its solubility is due to strong ion-dipole interactions between the ions and water molecules. It's a relatively stable compound under normal conditions, but can decompose under high temperatures or in the presence of certain reducing agents.
Key Properties of NH₄NO₃:
- Molar Mass: Approximately 80.04 g/mol
- Solubility: Highly soluble in water
- Appearance: White crystalline solid
- Applications: Fertilizer, explosive (in specific formulations)
Practical Applications and Considerations
While the reaction itself might not have widespread direct applications, understanding this type of double displacement reaction is fundamental in several fields:
- Analytical Chemistry: This type of reaction is used in qualitative and quantitative analysis to identify and determine the concentration of ions in solution. The precipitation of lead(IV) phosphate could be used to determine the concentration of lead or phosphate ions in a sample.
- Environmental Chemistry: Understanding the solubility of compounds is crucial for managing environmental contamination. The low solubility of lead(IV) phosphate highlights the need for careful management of lead-containing compounds to prevent water pollution.
- Materials Science: The properties of lead(IV) phosphate might find niche applications in specific material synthesis, although its toxicity limits its widespread use.
Safety Precautions
Working with lead compounds requires extreme caution due to their toxicity. Lead poisoning can cause serious health problems, affecting the nervous system, kidneys, and reproductive system. The following safety precautions are essential:
- Proper Ventilation: Always conduct the experiment in a well-ventilated area to avoid inhaling lead dust or fumes.
- Personal Protective Equipment (PPE): Wear appropriate PPE, including gloves, eye protection, and a lab coat, to prevent direct contact with lead compounds.
- Waste Disposal: Dispose of lead-containing waste according to the appropriate local regulations. Never dispose of lead-containing waste in regular trash.
Conclusion
The reaction between ammonium phosphate and lead(IV) nitrate is a complex yet insightful example of a double displacement reaction. It illustrates the importance of solubility rules in predicting reaction outcomes and highlights the need for careful handling of toxic substances. Understanding this reaction provides a foundation for exploring various aspects of chemistry, including stoichiometry, solubility, and the properties of different ionic compounds. The detailed analysis of reactants and products, coupled with the safety considerations, offers a comprehensive perspective on this specific reaction and its relevance in different scientific domains. Further research into the specific properties and potential applications of lead(IV) phosphate, while acknowledging its toxicity, may unveil even more interesting aspects of this chemical process.
Latest Posts
Latest Posts
-
What Is The Valence Value Of Carbon
Mar 30, 2025
-
These Neurons Transmit Impulses From Cns To Effectors
Mar 30, 2025
-
Place The Following Parts Of A Reflex Arc In Order
Mar 30, 2025
-
Least Common Denominator Of Rational Expressions Calculator
Mar 30, 2025
-
What Is The Least Common Multiple Of 25 And 30
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about Nh4 3po4 Pb No3 4 Pb3 Po4 4 Nh4no3 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
