What Is The Valence Value Of Carbon
Juapaving
Mar 30, 2025 · 6 min read
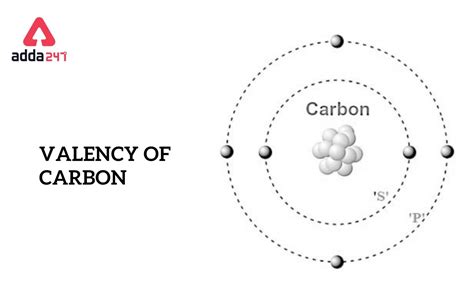
Table of Contents
What is the Valence Value of Carbon? Unlocking the Key to Organic Chemistry
Carbon. The very word conjures images of complex molecules, the building blocks of life, and the foundation of organic chemistry. But at the heart of this incredible versatility lies a simple, yet profound, characteristic: its valence. Understanding the valence value of carbon is crucial to comprehending the vast array of molecules it can form, from simple hydrocarbons to the intricate biomolecules that make up living organisms. This article delves deep into the valence of carbon, exploring its implications and significance in various fields.
Understanding Valence: The Bonding Power of Atoms
Before we delve into the specifics of carbon, let's establish a clear understanding of valence itself. Valence refers to the combining capacity of an atom. It essentially represents the number of electrons an atom can either gain, lose, or share to achieve a stable electron configuration, usually a full outer electron shell. This stable configuration, often resembling that of a noble gas, is thermodynamically favorable, driving atoms to form chemical bonds.
The Octet Rule: The Driving Force Behind Bonding
The octet rule is a crucial guideline in understanding valence. It states that atoms tend to gain, lose, or share electrons to achieve eight electrons in their outermost electron shell (valence shell). This configuration mimics the stable electron arrangement of noble gases, making it energetically favorable. There are exceptions to the octet rule, particularly with elements beyond the second period, but it serves as a valuable framework for understanding the bonding behavior of many elements, including carbon.
The Valence of Carbon: Four is the Magic Number
Carbon, with its atomic number 6, possesses six electrons. Its electron configuration is 1s²2s²2p². This means it has four electrons in its outermost shell (the second shell). To achieve a stable octet, carbon needs four more electrons. Therefore, the valence of carbon is 4. This seemingly simple number is the key to carbon's extraordinary ability to form a vast range of compounds.
Carbon's Bonding Prowess: Single, Double, and Triple Bonds
Carbon's valence of 4 allows it to form four covalent bonds. A covalent bond involves the sharing of electrons between atoms. Carbon can achieve its octet by forming:
- Single bonds: Sharing one electron pair with another atom. A classic example is methane (CH₄), where carbon forms four single bonds with four hydrogen atoms.
- Double bonds: Sharing two electron pairs with another atom. Ethylene (C₂H₄) exemplifies this, with each carbon atom forming a double bond with the other carbon atom and two single bonds with hydrogen atoms.
- Triple bonds: Sharing three electron pairs with another atom. Acetylene (C₂H₂) showcases this, featuring a triple bond between the two carbon atoms and a single bond to each hydrogen atom.
This ability to form single, double, and triple bonds, along with the capacity to form chains and rings, is what makes carbon so unique and versatile.
The Significance of Carbon's Valence: Implications Across Disciplines
The valence of carbon has profound implications across various scientific disciplines, shaping our understanding of:
1. Organic Chemistry: The Chemistry of Life
The valence of carbon is the cornerstone of organic chemistry, the study of carbon-containing compounds. The incredible diversity of organic molecules stems directly from carbon's ability to form four bonds, creating long chains, branched structures, and complex ring systems. This forms the basis of:
- Hydrocarbons: The simplest organic compounds, consisting solely of carbon and hydrogen atoms. These range from simple alkanes like methane to complex aromatic hydrocarbons like benzene.
- Functional groups: Atoms or groups of atoms that impart specific chemical properties to organic molecules. These functional groups, attached to carbon backbones, dictate the reactivity and behavior of the molecule. Examples include hydroxyl (-OH), carboxyl (-COOH), and amino (-NH₂) groups.
- Biomolecules: The essential molecules of life, including carbohydrates, lipids, proteins, and nucleic acids. The carbon backbone is central to the structure and function of each of these biomolecules. For example, the sugar molecules in DNA and RNA have carbon backbones, forming the framework for the genetic code.
2. Materials Science: Designing Novel Materials
Understanding carbon's valence is crucial in materials science for the design and synthesis of novel materials. The ability to manipulate the bonding of carbon atoms enables the creation of:
- Polymers: Large molecules composed of repeating structural units. Many synthetic polymers, such as plastics and rubbers, rely on carbon's ability to form long chains.
- Nanomaterials: Materials with structures at the nanoscale, exhibiting unique properties not observed in their bulk counterparts. Carbon nanotubes and graphene, both composed of carbon atoms arranged in specific configurations, are prime examples of the remarkable properties attainable through controlled manipulation of carbon's bonding. These materials are being explored for applications in electronics, energy storage, and medicine.
3. Biochemistry: Understanding Biological Processes
In biochemistry, the valence of carbon is fundamental to understanding the intricate workings of living organisms. Carbon's ability to form diverse molecules allows it to:
- Store energy: Carbohydrates store energy in the form of glucose and other sugar molecules, all based on carbon backbones.
- Construct structural components: Proteins, the workhorses of cells, are built from amino acids linked together via peptide bonds, with carbon forming the backbone of the amino acid structure.
- Transmit genetic information: DNA and RNA, carrying the genetic code, rely on a carbon backbone to hold the nucleotide bases in sequence.
Exceptions to the Octet Rule and Carbon's Versatility
While the octet rule serves as a valuable guideline, there are exceptions, and carbon displays some intriguing variations:
- Carbocations and Carbanions: These are charged carbon species that violate the octet rule, possessing either a positive (carbocation) or negative (carbanion) charge, resulting in only three or five bonds respectively. They are highly reactive intermediates in many organic reactions.
- Free Radicals: These contain unpaired electrons, giving them high reactivity. Carbon-centered free radicals are crucial intermediates in various chemical reactions, often initiating chain reactions.
Conclusion: Carbon's Enduring Importance
The valence of carbon, a seemingly simple numerical value, is the bedrock of an incredibly vast and complex world of chemistry. Its ability to form four bonds, along with its capacity to form single, double, and triple bonds, underpins the diversity of organic molecules, the functionality of biomolecules, and the design of novel materials. From the simplest hydrocarbons to the complex intricacies of DNA, carbon's valence of 4 remains a fundamental principle driving the world around us. Further exploration into the nuances of carbon's bonding will undoubtedly continue to unlock new scientific advancements and technological possibilities. Understanding this fundamental aspect of carbon chemistry is essential for anyone seeking to delve into the fascinating world of organic chemistry, biochemistry, and materials science.
Latest Posts
Latest Posts
-
How Much Sides Does A Octagon Have
Apr 01, 2025
-
The Galapagos Finch Species Are An Excellent Example Of
Apr 01, 2025
-
Is The Nucleolus A Plant Or Animal Cell
Apr 01, 2025
-
What Is True About Irrational Numbers
Apr 01, 2025
-
What Is The Lcm Of 4 5 And 8
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about What Is The Valence Value Of Carbon . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
