Is Condensation A Chemical Or Physical Change
Juapaving
Mar 31, 2025 · 5 min read
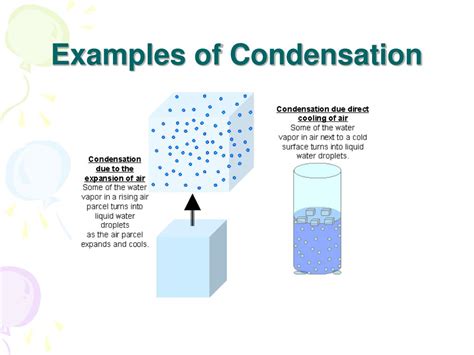
Table of Contents
Is Condensation a Chemical or Physical Change? A Deep Dive
Condensation, the process by which water vapor transforms into liquid water, is a ubiquitous phenomenon influencing weather patterns, everyday household occurrences, and even industrial processes. But is this transformation a chemical change, involving the alteration of molecular structure, or a physical change, affecting only the state of matter? This article will delve into the intricacies of condensation, exploring its nature, the underlying principles, and why it's definitively classified as a physical change.
Understanding the Difference Between Physical and Chemical Changes
Before we dissect condensation, let's establish a clear understanding of the difference between physical and chemical changes.
Physical Changes:
- No new substance is formed: The fundamental composition of the matter remains the same. Only the physical properties, such as shape, size, or state (solid, liquid, gas), might alter.
- Changes are often reversible: The original substance can be recovered through relatively simple means. For example, melting ice (physical change) can be reversed by refreezing it.
- Examples: Melting, freezing, boiling, condensation, dissolving (in some cases).
Chemical Changes:
- A new substance is formed: The molecular structure of the matter changes, resulting in a substance with different properties.
- Changes are usually irreversible: Recovering the original substance requires complex chemical reactions.
- Examples: Burning, rusting, cooking, digestion.
The Process of Condensation: A Microscopic View
Condensation occurs when water vapor, a gaseous state of water (H₂O), transitions into liquid water. Let's examine this process at a molecular level:
- Water vapor: In its gaseous state, water molecules are widely dispersed and possess high kinetic energy, allowing them to move freely and rapidly. The weak intermolecular forces (hydrogen bonds) between water molecules are easily overcome.
- Cooling and energy loss: When water vapor comes into contact with a cooler surface (or the surrounding air cools), the water molecules lose kinetic energy.
- Intermolecular forces take over: As kinetic energy decreases, the intermolecular forces between water molecules become more significant. These forces pull the molecules closer together.
- Liquid water formation: Once enough molecules have slowed down and clustered together, they form liquid water droplets. The molecules are still H₂O; their chemical structure hasn't changed – only their arrangement and energy state.
Why Condensation is a Physical Change
The key to understanding why condensation is a physical change lies in the absence of a change in the chemical composition of water. Throughout the entire process:
- The molecules remain the same: The water molecules (H₂O) retain their original chemical structure. No new chemical bonds are formed, and no existing bonds are broken.
- The process is reversible: Liquid water can be easily converted back into water vapor through evaporation or boiling. This reversibility is a hallmark of a physical change.
- No new substance is created: The liquid water formed through condensation is still water, possessing the same chemical properties as the initial water vapor.
Examples of Condensation in Everyday Life
Condensation is a commonplace phenomenon, influencing various aspects of our daily lives:
1. Dew formation:
The cooling of the Earth's surface during the night causes water vapor in the air to condense, forming tiny water droplets on grass, leaves, and other surfaces.
2. Fog and clouds:
Fog and clouds are formed when water vapor in the air condenses around microscopic particles, like dust or pollen. As more water vapor condenses, the droplets grow larger, becoming visible as fog or clouds.
3. Water droplets on a cold glass:
When a cold glass is placed in a humid environment, the water vapor in the air condenses on the cooler surface of the glass, forming visible droplets. This is a simple and readily observable demonstration of condensation.
4. Steam from a hot shower:
The hot water vapor from a shower encounters the cooler air in the bathroom, leading to condensation and the formation of visible steam.
5. Breath on a cold day:
The warm, moist air exhaled from our lungs condenses in the colder air, forming a visible cloud of tiny water droplets.
Condensation in Industrial Processes
Condensation also plays a crucial role in various industrial processes:
1. Desalination:
Condensation is a key step in desalination processes, where seawater is evaporated and then condensed to produce fresh water.
2. Power plants:
In power plants, condensation of steam is used to generate electricity. The steam, after passing through turbines, is condensed back into liquid water, completing the cycle.
3. Refrigeration:
Refrigeration systems utilize condensation to remove heat. The refrigerant gas, after absorbing heat, condenses into a liquid, releasing the absorbed heat.
Addressing Common Misconceptions
Some might confuse condensation with processes involving chemical changes. It's important to differentiate:
- Condensation vs. Chemical Reactions: While some chemical reactions might produce water as a byproduct, condensation itself is a purely physical change. The formation of water from hydrogen and oxygen is a chemical reaction, but the condensation of already-existing water vapor is physical.
- Condensation vs. Precipitation: Precipitation (rain, snow, etc.) is the result of condensation in the atmosphere, but the condensation itself is the phase change, not the subsequent falling of water droplets.
Conclusion: A Definitive Physical Change
In conclusion, condensation is unequivocally a physical change. The process involves a change in the state of water from gas to liquid, without altering the chemical composition of the water molecules. The reversibility of the process, the absence of new substance formation, and the preservation of the chemical structure of water all solidify this classification. Understanding this fundamental concept is crucial not only in scientific contexts but also in comprehending various everyday phenomena and industrial processes involving this vital phase transition. From the dew on a morning lawn to the sophisticated workings of power plants, condensation's role as a physical change remains constant and critical.
Latest Posts
Latest Posts
-
Is Coal And Charcoal The Same
Apr 01, 2025
-
What Are The Common Multiples Of 3 And 5
Apr 01, 2025
-
Lcm Of 2 And 3 And 6
Apr 01, 2025
-
What Is The Prime Factorization 81
Apr 01, 2025
-
No Name This Compound According To Iupac Nomenclature Rules Responses
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Is Condensation A Chemical Or Physical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
