Is Air A Compound Or Element Or Mixture
Juapaving
Mar 21, 2025 · 5 min read
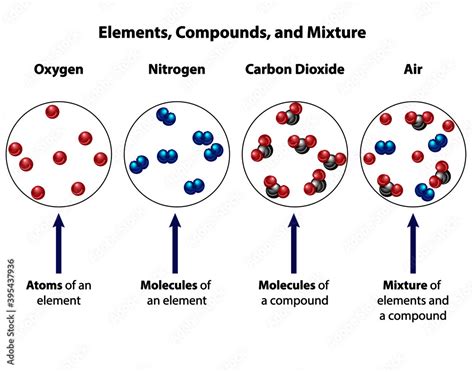
Table of Contents
Is Air a Compound, Element, or Mixture? A Deep Dive into Atmospheric Composition
The question, "Is air a compound, element, or mixture?" seems simple at first glance. However, a comprehensive understanding requires delving into the intricacies of chemistry and atmospheric science. The answer, as we'll explore, is far more nuanced than a simple one-word response. This detailed exploration will examine the components of air, their interactions, and the scientific definitions that determine its classification. We'll also explore the implications of air's composition on various aspects of our lives and the planet.
Understanding the Basic Terminology
Before diving into the specifics of air's composition, let's clarify the key terms:
-
Element: A pure substance consisting only of atoms that all have the same numbers of protons in their atomic nuclei. Elements cannot be broken down into simpler substances by chemical means. Examples include oxygen (O), nitrogen (N), and hydrogen (H).
-
Compound: A substance formed when two or more chemical elements are chemically bonded together. These bonds create a new substance with properties distinct from its constituent elements. Water (H₂O) is a classic example of a compound, possessing properties vastly different from hydrogen and oxygen in their elemental forms.
-
Mixture: A substance comprising two or more components not chemically bonded. These components retain their individual chemical properties and can be separated by physical means, such as filtration, distillation, or evaporation. Air, as we'll see, falls into this category.
The Composition of Air: A Detailed Look
Air is a complex mixture of various gases, liquids (in the form of aerosols), and solids (dust particles). The primary components, however, are gases. The relative proportions of these gases vary slightly depending on location (altitude, proximity to industrial areas, etc.), but a general composition is as follows:
Major Components:
-
Nitrogen (N₂): Approximately 78% of Earth's atmosphere is nitrogen gas. While essential for life, nitrogen gas in its atmospheric form is largely inert, meaning it doesn't readily react with other substances. It plays a crucial role in biological processes through nitrogen fixation.
-
Oxygen (O₂): Making up roughly 21% of the atmosphere, oxygen is vital for respiration in most living organisms. It's highly reactive and participates in numerous chemical processes, including combustion and oxidation.
-
Argon (Ar): This inert noble gas constitutes about 0.93% of the atmosphere. Its inert nature means it doesn't participate in most chemical reactions.
Minor Components:
Besides the major components, air contains several other gases in trace amounts:
-
Carbon Dioxide (CO₂): Though present in relatively small amounts (around 0.04%), carbon dioxide plays a crucial role in the greenhouse effect, influencing Earth's climate. Its concentration is steadily increasing due to human activities.
-
Neon (Ne), Helium (He), Methane (CH₄), Krypton (Kr), Hydrogen (H₂), Nitrous Oxide (N₂O), Xenon (Xe), Ozone (O₃): These gases exist in even smaller concentrations, yet each plays specific roles in atmospheric processes and some have significant implications for climate change and air quality.
Variable Components:
The composition of air also includes components that vary significantly depending on location and time:
-
Water Vapor (H₂O): The amount of water vapor in the air can range from near zero to several percent, depending on temperature and humidity. It plays a crucial role in weather patterns and the water cycle.
-
Aerosols: These are tiny solid or liquid particles suspended in the air, including dust, pollen, sea salt, soot, and pollutants. Aerosols can impact air quality, cloud formation, and climate.
Why Air is a Mixture, Not a Compound
Given the presence of multiple elements and compounds, why isn't air classified as a compound? The answer lies in the nature of the interactions between the components:
-
No Chemical Bonds: The gases in air are not chemically bonded to each other. They are simply mixed together physically. This is crucial because compounds require the formation of chemical bonds to create a new substance with unique properties. Air retains the properties of its individual components.
-
Separable Components: The components of air can be separated using physical methods. For instance, liquefaction involves cooling air to extremely low temperatures, causing its components to condense into liquids at different points, allowing for their separation. This would be impossible with a true chemical compound.
-
Variable Composition: The composition of air varies depending on location and conditions. A true compound would have a fixed and constant chemical formula.
The Significance of Air's Composition
The composition of air isn't just a matter of scientific classification; it's fundamental to life on Earth and numerous other processes:
-
Respiration: Oxygen's presence is essential for aerobic respiration in most living organisms. Without it, life as we know it wouldn't exist.
-
Combustion: Oxygen is a key component in combustion reactions, which are vital for energy production in various applications, from power generation to cooking.
-
Climate Regulation: Gases like carbon dioxide, methane, and water vapor play crucial roles in regulating Earth's temperature through the greenhouse effect. Changes in their concentrations significantly impact global climate patterns.
-
Air Quality: The presence of pollutants and aerosols in the air significantly affects air quality, impacting human health and the environment.
-
Weather Patterns: Water vapor and its interactions with other atmospheric components are central to the formation of clouds, precipitation, and other weather phenomena.
Conclusion: Air – A Vital Mixture
In conclusion, air is definitively a mixture, not a compound or an element. Its composition is a complex blend of gases, liquids, and solids, each playing a crucial role in various natural and human-influenced processes. Understanding the intricacies of air's composition is essential for comprehending climate change, air quality, and the very foundation of life on Earth. The seemingly simple question of air's classification opens a window into the fascinating world of atmospheric science and chemistry, highlighting the interconnectedness of various scientific disciplines and their impact on our planet. Further research into specific components, such as the impact of increasing CO2 levels, or the role of aerosols in climate modelling, only deepens our appreciation for this vital mixture that sustains us. The ongoing study of air composition is paramount for addressing environmental challenges and securing a sustainable future.
Latest Posts
Latest Posts
-
Highest Common Factor Of 36 And 84
Mar 28, 2025
-
What Is Mcml In Roman Numerals
Mar 28, 2025
-
Which Of The Following Is The Final Product Of Spermiogenesis
Mar 28, 2025
-
Which Of The Following Is An Example Of A Combination
Mar 28, 2025
-
Homologous Chromosomes Align On The Equator During
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Is Air A Compound Or Element Or Mixture . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
