Homologous Chromosomes Align On The Equator During
Juapaving
Mar 28, 2025 · 6 min read
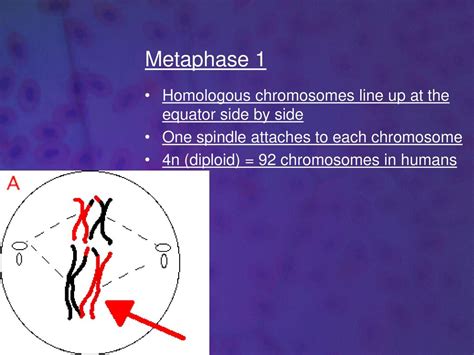
Table of Contents
- Homologous Chromosomes Align On The Equator During
- Table of Contents
- Homologous Chromosomes Align on the Equator During Meiosis I: A Deep Dive into Metaphase I
- The Significance of Homologous Chromosome Alignment
- The Stages Leading Up to Metaphase I: A Step-by-Step Approach
- The Molecular Mechanisms Driving Metaphase I Alignment
- Consequences of Errors in Metaphase I
- Metaphase I and Genetic Diversity: A Summary
- Future Research Directions
- Latest Posts
- Latest Posts
- Related Post
Homologous Chromosomes Align on the Equator During Meiosis I: A Deep Dive into Metaphase I
Meiosis, the specialized cell division process that produces gametes (sperm and egg cells), is crucial for sexual reproduction. A key stage in meiosis is Metaphase I, where homologous chromosomes align along the metaphase plate (the cell's equator). This precise arrangement is fundamental to the successful segregation of homologous chromosomes, ensuring genetic diversity in offspring. Understanding the intricacies of Metaphase I is critical to comprehending the entire meiotic process and its implications for inheritance.
The Significance of Homologous Chromosome Alignment
Before delving into the mechanics of Metaphase I, let's establish the importance of aligning homologous chromosomes. Homologous chromosomes are pairs of chromosomes, one inherited from each parent. They carry the same genes in the same order, but may have different versions (alleles) of those genes. This variation is the basis of genetic diversity.
During Metaphase I, the precise alignment of these homologous chromosome pairs is paramount for two crucial reasons:
-
Independent Assortment: The random orientation of homologous chromosome pairs along the metaphase plate leads to independent assortment. This means that the maternal and paternal chromosomes of each homologous pair are randomly distributed to the daughter cells. This random distribution generates a vast number of possible chromosome combinations in the resulting gametes. For example, in humans with 23 pairs of chromosomes, the number of possible chromosome combinations is 2<sup>23</sup>, contributing significantly to genetic diversity within a population.
-
Crossing Over: The physical proximity of homologous chromosomes during Metaphase I facilitates crossing over, or recombination. Crossing over is the exchange of genetic material between non-sister chromatids of homologous chromosomes. This process shuffles alleles between homologous chromosomes, creating new combinations of alleles on each chromosome. Crossing over significantly increases genetic diversity beyond what is achieved through independent assortment alone. The resulting chromosomes are unique combinations of maternal and paternal DNA, further enhancing the genetic variability in the offspring.
The Stages Leading Up to Metaphase I: A Step-by-Step Approach
Understanding Metaphase I requires appreciating the events that precede it. The meiotic process is a continuous one, with distinct stages that build upon each other.
1. Prophase I: This is a protracted and complex stage where homologous chromosomes pair up, forming structures called bivalents or tetrads. Within the bivalents, non-sister chromatids intertwine, allowing for crossing over to occur at points called chiasmata. Prophase I encompasses several sub-stages: leptotene, zygotene, pachytene, diplotene, and diakinesis, each characterized by specific chromosomal changes and configurations.
2. Prometaphase I: The nuclear envelope breaks down, and the spindle fibers, originating from the centrosomes at opposite poles of the cell, begin to attach to the kinetochores of the chromosomes. These kinetochores are protein structures located at the centromere of each chromosome and serve as attachment points for the spindle fibers.
3. Metaphase I: This is where homologous chromosomes, now attached to spindle fibers from opposite poles, align along the metaphase plate. This alignment is crucial because it ensures that each homologous chromosome pair will segregate correctly during anaphase I. The precise positioning of the chromosomes on the metaphase plate is regulated by a complex interplay of molecular motors and microtubules.
The Molecular Mechanisms Driving Metaphase I Alignment
The accurate alignment of homologous chromosomes during Metaphase I is a meticulously orchestrated process involving several key molecular players:
-
Cohesins: These protein complexes hold sister chromatids together, ensuring that they remain attached throughout meiosis I. Cohesins are particularly important in ensuring proper chromosome alignment. The regulated removal of cohesins along chromosome arms is crucial for the separation of homologous chromosomes during anaphase I, while cohesin at the centromere is maintained to hold sister chromatids together until anaphase II.
-
Kinetochore Microtubules: These microtubules extend from the centrosomes and attach to the kinetochores of each chromosome. The dynamic attachment and detachment of kinetochore microtubules are crucial for chromosome movement and alignment. The balance of forces exerted by the microtubules pulling from opposite poles ensures the correct positioning of the chromosomes.
-
Motor Proteins: Various motor proteins, including kinesins and dyneins, contribute to the movement of chromosomes along the spindle fibers. These motor proteins actively “walk” along the microtubules, using ATP as an energy source to generate the forces necessary for chromosome movement and alignment.
-
Chromosome Condensation: The highly condensed state of chromosomes in Metaphase I facilitates their efficient manipulation by the spindle apparatus. The level of condensation is tightly regulated throughout meiosis to ensure proper chromosome alignment and segregation.
-
Spindle Checkpoint: A crucial quality control mechanism, the spindle checkpoint ensures that all chromosomes are correctly attached to the spindle fibers before anaphase I begins. This checkpoint prevents premature chromosome segregation, which could lead to aneuploidy (an abnormal number of chromosomes in the daughter cells). The spindle checkpoint delays the progression of meiosis until all chromosomes are correctly aligned.
Consequences of Errors in Metaphase I
Errors during Metaphase I can have severe consequences, leading to genetic abnormalities in the resulting gametes and ultimately affecting the offspring. Such errors can manifest in several ways:
-
Nondisjunction: This is the failure of homologous chromosomes to separate properly during anaphase I. Nondisjunction can lead to gametes with an abnormal number of chromosomes, a condition known as aneuploidy. Examples include Down syndrome (trisomy 21), Turner syndrome (monosomy X), and Klinefelter syndrome (XXY).
-
Chromosome Aberrations: Errors during crossing over in Prophase I or improper chromosome segregation in Metaphase I can lead to structural chromosome aberrations such as deletions, duplications, inversions, and translocations. These aberrations can result in various genetic disorders and developmental abnormalities.
-
Infertility: Errors in meiosis can also lead to infertility, as gametes with abnormal chromosome numbers or structures may not be viable or capable of fertilization.
Metaphase I and Genetic Diversity: A Summary
Metaphase I is a pivotal stage in meiosis, playing a critical role in generating genetic diversity. The precise alignment of homologous chromosomes along the metaphase plate, driven by a complex interplay of molecular mechanisms, ensures the successful segregation of these chromosomes. Independent assortment and crossing over, both facilitated by this alignment, generate immense genetic variability in the resulting gametes. This genetic variation is fundamental for adaptation and evolution, ensuring the survival and propagation of species. Any disruption of the intricate processes involved in Metaphase I can lead to significant genetic abnormalities, emphasizing the vital role of this stage in maintaining genetic integrity.
Future Research Directions
Further research is needed to fully understand the intricacies of Metaphase I. Areas of ongoing investigation include:
-
Detailed mechanisms of spindle assembly and chromosome attachment: Understanding the precise molecular mechanisms that regulate the attachment of kinetochore microtubules to chromosomes is essential for a comprehensive understanding of chromosome segregation.
-
Regulation of cohesin removal: The precise timing and mechanism of cohesin removal are critical for accurate chromosome segregation. Further research is needed to elucidate these processes in detail.
-
Role of epigenetic modifications: Epigenetic modifications, such as DNA methylation and histone modifications, may play a role in regulating chromosome segregation during meiosis. Investigation into these epigenetic influences is an active area of research.
-
Development of novel therapeutic strategies for aneuploidy: Understanding the causes of nondisjunction is crucial for developing therapeutic strategies to prevent or correct aneuploidy-related disorders.
In conclusion, the alignment of homologous chromosomes on the equator during Metaphase I is a fundamental process in meiosis, crucial for genetic diversity and reproductive success. Continued research into the molecular mechanisms involved will provide further insights into this critical stage of cell division and its implications for human health and evolution.
Latest Posts
Latest Posts
-
A Ray Has Two Endpoints True Or False
Mar 31, 2025
-
A Substance That Cannot Be Broken Down By Chemical Means
Mar 31, 2025
-
Do Both Prokaryotic And Eukaryotic Cells Have Ribosomes
Mar 31, 2025
-
Which Law Represents A Balanced Chemical Equation
Mar 31, 2025
-
How To Calculate Tension In A Cable
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Homologous Chromosomes Align On The Equator During . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
