Hydrogen Metal Or Nonmetal Or Metalloid
Juapaving
Mar 28, 2025 · 5 min read
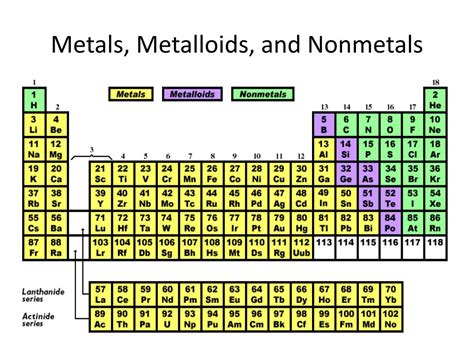
Table of Contents
Hydrogen: Metal, Nonmetal, or Metalloid? A Deep Dive into its Unique Properties
Hydrogen, the simplest element on the periodic table, is often presented as a nonmetal in introductory chemistry courses. However, the reality is far more nuanced. Its behavior defies easy categorization, leading to ongoing debate and research regarding its true nature. This comprehensive exploration will delve into the properties of hydrogen, examining the arguments for its classification as a nonmetal, a metal, and even a metalloid, ultimately concluding with a balanced perspective of this unique element.
The Case for Hydrogen as a Nonmetal
The most common classification of hydrogen is as a nonmetal. This categorization is primarily based on its observed properties under standard conditions:
1. Gaseous State at Room Temperature:
Unlike metals, which are typically solid at room temperature (exceptions exist), hydrogen exists as a diatomic gas (H₂). This gaseous nature is a key characteristic of nonmetals.
2. Low Density and Low Melting/Boiling Points:
Hydrogen possesses an exceptionally low density and extremely low melting and boiling points compared to metals. These properties align with those of nonmetals, further supporting its nonmetallic classification.
3. Poor Electrical and Thermal Conductivity:
Hydrogen exhibits poor electrical and thermal conductivity, which is a typical feature of nonmetals. Metals, in contrast, are excellent conductors of both electricity and heat.
4. Non-Metallic Reactivity:
Hydrogen readily forms covalent bonds with other nonmetals, demonstrating its nonmetallic reactivity. For example, its reaction with oxygen to form water (H₂O) is a classic example of covalent bonding. While it can form ionic compounds with highly electronegative elements like halogens, its behavior in these cases is still influenced by its nonmetallic characteristics.
5. Position on the Periodic Table:
Traditionally, hydrogen's placement at the top of Group 1 (alkali metals) or Group 17 (halogens) reflects its unique characteristics. Its placement is often debated. While it shares some similarities with alkali metals (losing one electron to achieve a noble gas configuration), it also resembles halogens (gaining one electron). Neither placement perfectly encapsulates its properties, solidifying the ambiguous nature of its position in the periodic table.
The Argument for Hydrogen as a Metal: High-Pressure Behavior
The perception of hydrogen as solely a nonmetal is challenged by its behavior under extreme conditions, particularly at high pressures. Under immense pressure, hydrogen demonstrates properties more akin to metals:
1. Metallic Hydrogen: A Theoretical Prediction
Theoretical calculations and simulations predict that at sufficiently high pressures (estimated to be around 400 GPa), hydrogen will undergo a phase transition to a metallic state. In this state, its electrons would become delocalized, allowing for the high electrical conductivity characteristic of metals.
2. Evidence from Experiments: A Shifting Paradigm
While creating and maintaining such extreme pressures in the laboratory is exceptionally challenging, experimental evidence is gradually accumulating to support the possibility of metallic hydrogen. Research involving shock compression techniques has shown some indicators of metallic behavior in hydrogen under extreme pressures, although definitive proof remains elusive.
3. Superconductivity Predictions: A Game-Changer
A further compelling argument for metallic hydrogen comes from predictions about its potential for superconductivity at relatively high temperatures. If achieved, this would have revolutionary implications for energy transmission and storage, further bolstering the importance of researching this exotic state of matter.
Exploring the Metalloid Argument: A Unique Position
Some argue that hydrogen's unique properties position it not as a simple metal or nonmetal, but as a metalloid. Metalloids, also known as semimetals, occupy a middle ground between metals and nonmetals, exhibiting properties of both.
1. Ambiguous Conductivity: A Defining Feature
Hydrogen's conductivity under standard conditions is poor, aligning with nonmetals. However, its predicted metallic behavior under high pressure suggests a transition in conductivity, blurring the lines between metals and nonmetals. This ambiguous conductivity is a hallmark of metalloids.
2. Varying Reactivity: A Metalloid Characteristic
Hydrogen’s reactivity varies depending on the conditions and the reacting element. While it readily forms covalent bonds with nonmetals, its reaction with metals to form metal hydrides presents a different, often ionic, interaction. This variation in reactivity aligns with the diverse behavior observed in metalloids.
3. Hydrogen's Unique Position: Bridge between Extremes
The ability of hydrogen to function as both an oxidizing agent (gaining electrons) and a reducing agent (losing electrons) further strengthens the metalloid argument. This duality underscores its unique position, bridging the gap between the typically electron-donating metals and electron-accepting nonmetals.
Conclusion: A Multifaceted Element Defying Simple Classification
In conclusion, simply classifying hydrogen as a metal, nonmetal, or metalloid is an oversimplification. Its behavior is profoundly influenced by the surrounding conditions. Under standard conditions, its properties align more closely with nonmetals. However, the theoretical and increasingly experimental evidence for metallic hydrogen under high pressure significantly complicates this straightforward classification. The possibility of a metallic phase, coupled with its ambiguous conductivity and reactivity, suggests that hydrogen possesses characteristics that blur the traditional lines between metals and nonmetals, making a strong case for its consideration as a unique element that defies simple categorization. Further research into its high-pressure behavior is critical to fully understand this enigmatic element and its potential to revolutionize various technological fields.
Future Directions and Research
Further research into metallic hydrogen is crucial for several reasons:
- Understanding Phase Transitions: A deeper understanding of the pressure-temperature conditions necessary for the phase transition to metallic hydrogen is needed.
- Superconductivity Exploration: Confirming the predictions of high-temperature superconductivity in metallic hydrogen would have profound scientific and technological implications.
- Material Science Applications: If metallic hydrogen can be stabilized, it could revolutionize material science and energy storage technologies.
- Planetary Science Implications: Understanding hydrogen's behavior under extreme conditions is important for comprehending the interiors of gas giant planets like Jupiter and Saturn, where metallic hydrogen is believed to exist.
The ongoing research into hydrogen’s unique properties promises to unravel many of the mysteries surrounding this simplest yet most complex element, potentially leading to scientific breakthroughs with far-reaching consequences. The debate over its classification will continue, highlighting the ever-evolving understanding of matter and its fascinating properties under varying conditions.
Latest Posts
Latest Posts
-
How Many Factors Does A Composite Number Have
Mar 31, 2025
-
What Is 4 The Square Root Of
Mar 31, 2025
-
Tendons And Ligaments Are Examples Of
Mar 31, 2025
-
What Is A Perfect Cube In Mathematics
Mar 31, 2025
-
What Is The Meaning Of Statutory Liquidity Ratio
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Hydrogen Metal Or Nonmetal Or Metalloid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
