How Many Valence Electrons Does P Have
Juapaving
Mar 06, 2025 · 5 min read
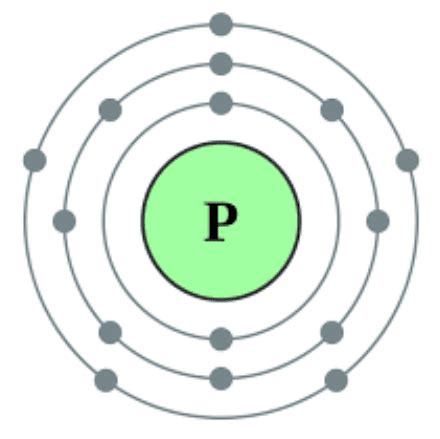
Table of Contents
How Many Valence Electrons Does Phosphorus (P) Have? A Deep Dive into Atomic Structure and Chemical Bonding
Phosphorus, a crucial element for life and numerous industrial applications, holds a fascinating place in the periodic table. Understanding its electronic structure, particularly the number of valence electrons, is key to comprehending its chemical behavior and reactivity. This article will delve deep into the question: how many valence electrons does phosphorus (P) have? We'll explore the concept of valence electrons, examine phosphorus's position in the periodic table, and investigate its implications for chemical bonding and the properties of phosphorus-containing compounds.
Understanding Valence Electrons
Before we pinpoint the number of valence electrons in phosphorus, let's establish a firm understanding of what valence electrons are. Valence electrons are the electrons located in the outermost shell of an atom. These electrons are crucial because they participate directly in chemical bonding. They determine an element's reactivity, its ability to form bonds with other atoms, and the types of bonds it forms (ionic, covalent, or metallic). The number of valence electrons significantly influences an element's chemical properties and how it behaves in chemical reactions.
The outermost shell is also known as the valence shell. The number of electrons in this shell dictates the atom's capacity to gain, lose, or share electrons to achieve a stable electron configuration, often referred to as the octet rule (eight electrons in the valence shell for most elements). This stability is a driving force in chemical reactions.
Phosphorus's Position in the Periodic Table
Phosphorus (P) is located in Group 15 (or VA) of the periodic table. This group is also known as the pnictogens. The periodic table's organization is not arbitrary; it reflects the underlying electronic structure of the elements. Elements within the same group share similar valence electron configurations, which directly influences their chemical behavior.
The group number for main group elements (like phosphorus) directly indicates the number of valence electrons. Group 15 elements have 5 valence electrons. This fundamental piece of information answers our primary question: Phosphorus (P) has 5 valence electrons.
Electronic Configuration of Phosphorus
To further solidify our understanding, let's examine phosphorus's electronic configuration. The electronic configuration describes how electrons are distributed among the different energy levels and sublevels within an atom. Phosphorus's atomic number is 15, meaning it has 15 protons and 15 electrons in a neutral atom. Its electronic configuration is:
1s² 2s² 2p⁶ 3s² 3p³
The outermost shell is the third shell (n=3), which contains the 3s and 3p sublevels. Adding the electrons in these sublevels (2 from 3s and 3 from 3p) gives us a total of 5 valence electrons. This confirms our earlier assertion based on phosphorus's group number in the periodic table.
Implications of 5 Valence Electrons for Phosphorus's Chemistry
The presence of 5 valence electrons profoundly impacts phosphorus's chemical behavior and the types of compounds it forms. Phosphorus can achieve a stable octet (eight valence electrons) in several ways:
- Gaining three electrons: This would result in the formation of a phosphide anion (P³⁻), which is commonly observed in ionic compounds with highly electropositive metals like sodium (Na₃P).
- Sharing electrons: This is more common for phosphorus. It can form three single covalent bonds or one triple bond and one single bond, resulting in various phosphorus-containing molecules like phosphine (PH₃) and phosphorus trichloride (PCl₃).
The ability to form multiple bonds explains the diverse range of phosphorus compounds, exhibiting varying degrees of oxidation states. This versatility extends into the complexity of its inorganic and organic chemistry.
Examples of Phosphorus Compounds and Bonding
Let's examine a few examples to illustrate how phosphorus's five valence electrons influence its bonding:
Phosphine (PH₃)
In phosphine, phosphorus forms three single covalent bonds with three hydrogen atoms. Each hydrogen atom contributes one electron, and phosphorus shares three of its five valence electrons, resulting in a stable octet for phosphorus and a duet (two electrons) for each hydrogen atom.
Phosphorus Trichloride (PCl₃)
Similarly, in phosphorus trichloride, phosphorus shares three of its valence electrons with three chlorine atoms, each contributing one electron to form three covalent bonds. The remaining two valence electrons on phosphorus remain as a lone pair.
Phosphorus Pentachloride (PCl₅)
Phosphorus penta chloride demonstrates phosphorus's capacity for exceeding the octet rule. Here, phosphorus forms five covalent bonds with five chlorine atoms. This expanded octet is possible due to the availability of d orbitals in the third shell, allowing for additional electron accommodation beyond the s and p orbitals.
Phosphorus in Biological Systems
The significance of phosphorus extends far beyond the laboratory. Phosphorus plays a crucial role in biological systems. It is a vital component of:
- DNA and RNA: The phosphate backbone of DNA and RNA is essential for genetic information storage and transfer.
- ATP (Adenosine Triphosphate): ATP is the primary energy currency of cells, and its structure incorporates phosphate groups.
- Phospholipids: These lipids are crucial components of cell membranes.
The unique bonding capabilities of phosphorus, enabled by its five valence electrons, underpin the crucial biological roles it plays.
Beyond the Basics: Exploring More Complex Phosphorus Chemistry
Phosphorus's chemistry extends well beyond simple covalent compounds. The element also exhibits allotropy, existing in various forms, such as white phosphorus, red phosphorus, and black phosphorus, each with distinct physical and chemical properties. These different allotropes arise from variations in phosphorus's bonding arrangements.
Moreover, the study of organophosphorus compounds is a vast and dynamic field. These compounds, containing carbon-phosphorus bonds, play critical roles in various applications, including pesticides, herbicides, and flame retardants. Understanding the bonding principles governing these compounds relies heavily on appreciating phosphorus's five valence electrons and their participation in covalent bonding.
Conclusion: The Significance of Valence Electrons
In conclusion, phosphorus (P) possesses 5 valence electrons, a fact directly derived from its position in Group 15 of the periodic table and confirmed by its electronic configuration. This seemingly simple number holds profound implications for phosphorus's chemical behavior, reactivity, and its crucial role in various chemical compounds and biological systems. Understanding the concept of valence electrons and their significance in determining chemical properties is fundamental to mastering chemistry and comprehending the world around us. The versatility afforded by these five electrons is a testament to the element's remarkable significance in both natural and synthetic contexts. Further exploration into phosphorus chemistry reveals its continued importance in scientific advancements and technological applications.
Latest Posts
Latest Posts
-
What Are The Factors For 31
Mar 07, 2025
-
What Is The Prime Factorization Of 22
Mar 07, 2025
-
Is Ba A Metal Or Nonmetal
Mar 07, 2025
-
Words That Start With E That Are Positive
Mar 07, 2025
-
What Is The Smallest Unit Of Matter
Mar 07, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Does P Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
