Is Ba A Metal Or Nonmetal
Juapaving
Mar 07, 2025 · 5 min read
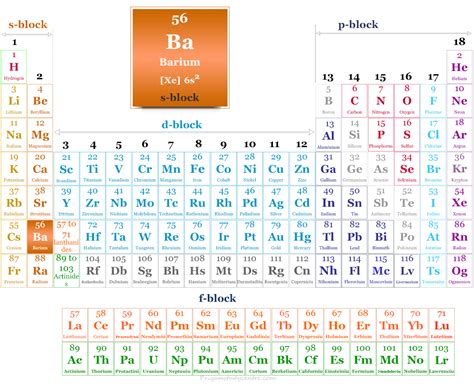
Table of Contents
Is Barium a Metal or a Nonmetal? A Deep Dive into Barium's Properties
Barium (Ba), element number 56 on the periodic table, is definitively a metal. Its properties firmly place it within the category of alkaline earth metals, a group known for its characteristic metallic behavior. This article will delve deep into the properties of barium, examining its physical and chemical characteristics to solidify its classification as a metal and dispel any confusion.
Understanding the Metal vs. Nonmetal Classification
Before we dive into the specifics of barium, let's establish a clear understanding of what differentiates metals from nonmetals. This distinction is based on several key properties:
Physical Properties:
- Luster: Metals generally possess a shiny, lustrous appearance. Nonmetals typically lack this characteristic.
- Conductivity: Metals are excellent conductors of electricity and heat. Nonmetals are poor conductors, often acting as insulators.
- Malleability and Ductility: Metals can be hammered into sheets (malleability) and drawn into wires (ductility). Nonmetals are generally brittle and lack these properties.
- Density: Metals generally have higher densities than nonmetals.
- Melting and Boiling Points: Metals usually have high melting and boiling points, while nonmetals tend to have lower ones.
Chemical Properties:
- Reactivity: Metals tend to readily lose electrons to form positive ions (cations). Nonmetals tend to gain electrons to form negative ions (anions).
- Oxidation States: Metals typically exhibit positive oxidation states, while nonmetals can exhibit both positive and negative oxidation states.
- Formation of Compounds: Metals readily react with nonmetals to form ionic compounds.
Barium: A Case Study in Metallic Properties
Let's examine how barium aligns with these criteria, firmly establishing its classification as a metal.
Physical Properties of Barium: Evidence of Metallic Character
- Appearance: Barium is a silvery-white metal with a slightly yellowish tint. This lustrous appearance is a hallmark of metals.
- Conductivity: Barium is a good conductor of both electricity and heat, a key characteristic of metals. Its conductivity stems from the readily available electrons in its outer shell, which can move freely throughout the metal lattice.
- Malleability and Ductility: While somewhat brittle at room temperature, barium exhibits malleability and ductility, especially at elevated temperatures, a testament to its metallic nature. The ability to deform without fracturing is a strong indicator of its metallic bonding.
- Density: Barium has a relatively high density (3.62 g/cm³), consistent with most metals.
- Melting and Boiling Points: Barium has a relatively high melting point (727 °C) and boiling point (1897 °C), further supporting its classification as a metal. These high points are a consequence of the strong metallic bonds holding the barium atoms together.
Chemical Properties of Barium: Reinforcing its Metallic Identity
- Reactivity: Barium is a highly reactive metal, readily losing its two valence electrons to form a +2 ion (Ba²⁺). This strong tendency to lose electrons is a defining feature of metals. Its reactivity is such that it readily reacts with oxygen in the air to form barium oxide (BaO).
- Oxidation State: Barium almost exclusively exhibits a +2 oxidation state, a common characteristic of metals. This consistent positive oxidation state demonstrates its preference for electron loss.
- Compound Formation: Barium readily forms ionic compounds with nonmetals, such as barium chloride (BaCl₂) and barium sulfate (BaSO₄). The formation of these ionic compounds is a clear indication of its metallic character. The transfer of electrons from barium to the nonmetal is the driving force behind the formation of these stable compounds.
Comparing Barium to Other Elements: Highlighting the Differences
To further illustrate barium's metallic character, let's compare it to some nonmetals:
- Oxygen (O): Oxygen is a nonmetal. It exists as a diatomic gas (O₂), has poor conductivity, is brittle, and tends to gain electrons to form anions (O²⁻). The stark contrast between the properties of barium and oxygen highlights the fundamental differences between metals and nonmetals.
- Chlorine (Cl): Chlorine is a nonmetal. It exists as a diatomic gas (Cl₂), is a poor conductor, is brittle, and readily gains electrons to form anions (Cl⁻). Similar to oxygen, chlorine lacks the metallic properties exhibited by barium.
- Carbon (C): Carbon exists in various allotropes (diamond, graphite, etc.), but none exhibit the high conductivity, malleability, and ductility characteristic of barium. Carbon's tendency to form covalent bonds rather than ionic bonds further distinguishes it from metals.
The Alkaline Earth Metal Family: Barium's Metallic Kin
Barium belongs to Group 2 of the periodic table, also known as the alkaline earth metals. This group consists of highly reactive metals characterized by their two valence electrons. Other members include beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), and radium (Ra). All these elements share similar metallic properties, reinforcing barium's placement within this metallic family.
Dispelling Common Misconceptions
Some misconceptions about barium's classification might arise due to its reactivity and certain compounds' properties. It's crucial to clarify these points:
- Reactivity doesn't negate metallic character: Barium's high reactivity is a characteristic of many metals, particularly those in the alkaline earth group. High reactivity is a chemical property and does not change its underlying physical nature as a metal.
- Compound properties are different from elemental properties: While some barium compounds might appear non-metallic, this doesn't reflect the properties of elemental barium. For instance, barium sulfate (BaSO₄) is an insoluble white powder used as a radiocontrast agent in medical imaging. Its properties as a compound are distinct from the metallic properties of elemental barium.
Conclusion: Barium is Unmistakably a Metal
The overwhelming evidence presented throughout this article clearly establishes barium as a metal. Its lustrous appearance, excellent conductivity, malleability (at higher temperatures), high density, high melting and boiling points, and its tendency to lose electrons to form positive ions all align perfectly with the defining characteristics of metals. Its placement within the alkaline earth metal family further reinforces its metallic nature. While its reactivity and the properties of its compounds might initially lead to confusion, a thorough understanding of its elemental properties firmly solidifies barium's classification as a metal, without a shadow of a doubt.
Latest Posts
Latest Posts
-
What Is Prime Factorization Of 63
Mar 09, 2025
-
What Are The Building Blocks Of All Matter
Mar 09, 2025
-
Type Of Inflorescence Is Represented S Typical Of Sunflowers
Mar 09, 2025
-
Product Of Two Irrational Numbers Is Always
Mar 09, 2025
-
Ordering Numbers From Least To Greatest
Mar 09, 2025
Related Post
Thank you for visiting our website which covers about Is Ba A Metal Or Nonmetal . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
