Compare And Contrast Endothermic And Exothermic
Juapaving
Mar 23, 2025 · 6 min read
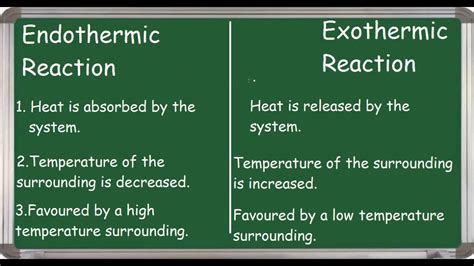
Table of Contents
Endothermic vs. Exothermic Reactions: A Comprehensive Comparison
Understanding the difference between endothermic and exothermic reactions is fundamental to grasping many concepts in chemistry and physics. While both involve energy transfer, the direction of that transfer dictates their classification and observable characteristics. This article provides a comprehensive comparison of endothermic and exothermic reactions, exploring their definitions, examples, applications, and the underlying principles governing them.
Defining Endothermic and Exothermic Reactions
At the core of understanding these reactions lies the concept of enthalpy (ΔH), a thermodynamic property representing the total heat content of a system. A change in enthalpy (ΔH) signifies the heat absorbed or released during a reaction at constant pressure.
Exothermic Reactions: These reactions release heat into their surroundings. The enthalpy of the products is lower than the enthalpy of the reactants (ΔH < 0). Think of it like this: the system is losing energy, and that energy manifests as heat given off to the environment. This often results in a noticeable increase in temperature.
Endothermic Reactions: These reactions absorb heat from their surroundings. The enthalpy of the products is higher than the enthalpy of the reactants (ΔH > 0). The system gains energy from its environment, resulting in a decrease in the surrounding temperature. You might feel a cooling effect during an endothermic process.
Key Differences Summarized
| Feature | Exothermic Reaction | Endothermic Reaction |
|---|---|---|
| Heat Transfer | Releases heat to surroundings | Absorbs heat from surroundings |
| Enthalpy Change (ΔH) | Negative (ΔH < 0) | Positive (ΔH > 0) |
| Temperature Change | Surroundings get warmer | Surroundings get cooler |
| Energy of Products | Lower than reactants | Higher than reactants |
| Feeling | Often feels warm or hot | Often feels cold or cool |
| Examples | Combustion, neutralization, condensation | Photosynthesis, melting ice, evaporating water |
Understanding Enthalpy Change (ΔH)
The enthalpy change (ΔH) is crucial in distinguishing between endothermic and exothermic processes. It's calculated as:
ΔH = H<sub>products</sub> - H<sub>reactants</sub>
Where:
- H<sub>products</sub> represents the total enthalpy of the products.
- H<sub>reactants</sub> represents the total enthalpy of the reactants.
A negative ΔH indicates an exothermic reaction (heat released), while a positive ΔH indicates an endothermic reaction (heat absorbed).
Examples of Exothermic Reactions
Exothermic reactions are abundant in everyday life and industrial processes. Here are some prominent examples:
1. Combustion: The burning of fuels like wood, natural gas (methane), propane, or gasoline is a classic example. These reactions release a significant amount of heat and light. The chemical bonds in the products (carbon dioxide and water) are stronger and lower in energy than those in the reactants (fuel and oxygen).
2. Neutralization Reactions: When an acid reacts with a base, a neutralization reaction occurs, typically releasing heat. For example, the reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH) produces sodium chloride (NaCl) and water (H₂O), along with a considerable amount of heat.
3. Condensation: The process of a gas turning into a liquid (condensation) is exothermic. The molecules in the liquid state have lower energy than in the gaseous state, releasing heat as they transition.
4. Respiration: Cellular respiration, the process by which living organisms convert glucose into energy, is an exothermic reaction. It releases energy in the form of ATP (adenosine triphosphate), along with heat, that sustains life processes.
5. Formation of ionic compounds: The formation of many ionic compounds from their constituent ions is exothermic. This is because the electrostatic attraction between the oppositely charged ions releases energy.
Examples of Endothermic Reactions
Endothermic reactions, while less visually striking than exothermic ones, are equally important in numerous natural and industrial processes:
1. Photosynthesis: Plants use sunlight energy to convert carbon dioxide and water into glucose and oxygen. This process absorbs energy from sunlight, making it endothermic.
2. Melting Ice: Melting ice requires energy input to break the hydrogen bonds holding the water molecules in a solid structure. The heat absorbed from the surroundings causes the ice to melt.
3. Evaporating Water: Similar to melting, converting liquid water to water vapor (evaporation) requires energy to overcome the intermolecular forces holding the liquid together. The heat absorbed causes the water to evaporate.
4. Decomposition Reactions: Many decomposition reactions, such as the breakdown of calcium carbonate (CaCO₃) into calcium oxide (CaO) and carbon dioxide (CO₂), are endothermic. These reactions require energy input to break the bonds in the reactant.
5. Cooking an Egg: Cooking an egg involves breaking and forming various chemical bonds. The net effect is an endothermic process requiring heat to denature the egg proteins.
Activation Energy: A Common Factor
Both endothermic and exothermic reactions require an initial input of energy known as activation energy (Ea). This energy is needed to break the existing bonds in the reactants, allowing new bonds to form and the reaction to proceed. Although exothermic reactions release more energy than they absorb overall (negative ΔH), they still need an initial energy boost to get started. Endothermic reactions require an input of energy throughout the entire process to achieve a positive ΔH.
Applications of Endothermic and Exothermic Reactions
The applications of endothermic and exothermic reactions are vast and span diverse fields:
Exothermic Reactions:
- Power Generation: Combustion in power plants generates electricity.
- Heating and Cooking: Burning fuels provides heat for homes and cooking.
- Industrial Processes: Many industrial processes, such as the production of cement and steel, rely on exothermic reactions.
- Welding: Exothermic reactions are utilized in welding processes to join metals.
- Hand warmers: These portable devices utilize exothermic reactions to generate heat.
Endothermic Reactions:
- Refrigeration: Endothermic reactions are used in refrigeration systems to absorb heat and cool the surroundings.
- Instant Cold Packs: These packs utilize endothermic reactions to quickly cool injuries.
- Some Industrial Processes: Certain industrial processes, such as the production of some fertilizers, involve endothermic reactions.
- Absorbing Excess Heat: In some industrial settings, endothermic reactions can be used to manage excessive heat generation in other processes.
Visualizing the Energy Changes
Energy diagrams graphically represent the energy changes during a reaction. For an exothermic reaction, the energy of the products is lower than the energy of the reactants, and the difference is released as heat. The graph shows a downward slope from reactants to products. Conversely, for an endothermic reaction, the energy of the products is higher than the energy of the reactants, and the difference is absorbed as heat. The graph displays an upward slope from reactants to products. Both diagrams include the activation energy barrier that must be overcome for the reaction to proceed.
Practical Applications and Considerations
The understanding of endothermic and exothermic reactions is crucial in various practical applications:
- Chemical Engineering: Designing efficient chemical processes often involves optimizing heat transfer, requiring a thorough understanding of enthalpy changes.
- Environmental Science: Understanding these reactions is vital in analyzing environmental impacts, such as the heat generated by combustion or the cooling effect of evaporation.
- Material Science: The development of new materials often relies on understanding the energy changes associated with chemical reactions.
- Food Science: Cooking, preservation, and the overall chemistry of food processing are heavily influenced by these principles.
Conclusion
Endothermic and exothermic reactions are fundamental concepts in chemistry, with diverse applications in various fields. While they represent opposite energy transfer directions, both are governed by the principles of thermodynamics and activation energy. Understanding the differences between these reaction types, their energy profiles, and their practical applications is crucial for advancing scientific knowledge and technological innovation across multiple disciplines. Further investigation into specific reaction mechanisms and their applications can lead to even greater insights into the intricate world of chemical transformations.
Latest Posts
Latest Posts
-
Select The Components Of A Nucleotide
Mar 25, 2025
-
Which Of The Following Statements About Vaccines Is True
Mar 25, 2025
-
What Is A 9 Sided Polygon
Mar 25, 2025
-
Carbonic Acid Strong Or Weak Acid
Mar 25, 2025
-
How To Convert Kilograms Into Grams
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Compare And Contrast Endothermic And Exothermic . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
