Carbonic Acid Strong Or Weak Acid
Juapaving
Mar 25, 2025 · 5 min read
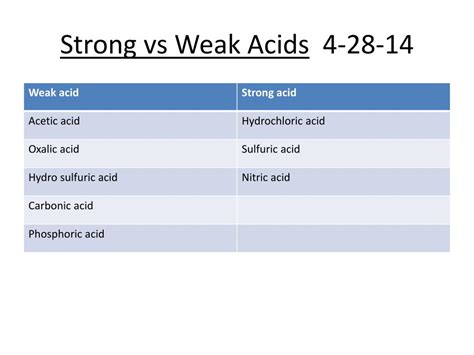
Table of Contents
Carbonic Acid: A Deep Dive into its Strength and Properties
Carbonic acid (H₂CO₃) is a ubiquitous chemical species found in various natural and industrial systems. Understanding its acidic nature, specifically whether it's a strong or weak acid, is crucial in many fields, from geology and oceanography to biology and chemistry. This comprehensive article will explore the properties of carbonic acid, explaining why it's classified as a weak acid and detailing its significant role in various processes. We'll delve into its dissociation constants, buffering capacity, and its impact on pH levels in different environments.
Is Carbonic Acid a Strong or Weak Acid? The Answer and Why it Matters
The simple answer is: carbonic acid is a weak acid. Unlike strong acids like hydrochloric acid (HCl) or sulfuric acid (H₂SO₄), which completely dissociate in water, carbonic acid only partially dissociates. This means that only a small fraction of H₂CO₃ molecules break down into their constituent ions (hydrogen ions, H⁺, and bicarbonate ions, HCO₃⁻) in an aqueous solution.
This seemingly minor difference has profound implications. The partial dissociation of carbonic acid leads to several crucial properties, including:
- A lower concentration of H⁺ ions: This directly affects the pH of a solution. Strong acids drastically lower pH, while weak acids cause a more moderate decrease.
- Buffering capacity: Carbonic acid, along with its conjugate base (bicarbonate), forms a crucial buffer system in biological systems and natural environments, helping to maintain a relatively stable pH.
- Equilibrium reactions: The incomplete dissociation of carbonic acid results in equilibrium reactions, which are governed by specific equilibrium constants.
Understanding Acid Strength: Dissociation and Equilibrium
The strength of an acid is directly related to its ability to donate protons (H⁺ ions). Strong acids readily donate protons, while weak acids only partially donate them. This difference is quantified using the acid dissociation constant (Ka).
The dissociation of carbonic acid in water can be represented by the following equilibrium reactions:
Reaction 1: H₂CO₃ ⇌ H⁺ + HCO₃⁻
Reaction 2: HCO₃⁻ ⇌ H⁺ + CO₃²⁻
Each reaction has its own dissociation constant:
- Ka₁ (for Reaction 1): This represents the dissociation of carbonic acid into bicarbonate and hydrogen ions. Its value is approximately 4.3 x 10⁻⁷.
- Ka₂ (for Reaction 2): This represents the dissociation of bicarbonate into carbonate and hydrogen ions. Its value is approximately 4.8 x 10⁻¹¹.
The relatively small values of Ka₁ and Ka₂ confirm that carbonic acid is a weak acid. A higher Ka value indicates a stronger acid. The much smaller value of Ka₂ compared to Ka₁ shows that the second dissociation is even less likely to occur than the first.
The Role of Carbonic Acid in Biological Systems
Carbonic acid plays a vital role in maintaining the acid-base balance in biological systems. The carbonic acid-bicarbonate buffer system is crucial for regulating blood pH in humans and other animals. This system works by resisting changes in pH caused by the addition of acids or bases. When excess H⁺ ions are present, bicarbonate ions react to form carbonic acid, minimizing the pH drop. Conversely, when excess hydroxide ions (OH⁻) are present, carbonic acid reacts, neutralizing the base and preventing a significant pH increase.
The Carbonic Anhydrase Enzyme: A Key Player
The efficient conversion between carbon dioxide (CO₂), water (H₂O), and carbonic acid is facilitated by the enzyme carbonic anhydrase. This enzyme dramatically accelerates the rate of these reactions, ensuring that the buffer system operates effectively. Without carbonic anhydrase, the conversion would be too slow to maintain proper pH homeostasis.
Carbonic Acid in the Environment: Ocean Acidification
Carbonic acid's role extends beyond biology. It's a major player in the Earth's carbon cycle and plays a crucial role in ocean chemistry. Ocean acidification, a significant environmental concern, is directly linked to increased atmospheric CO₂. As CO₂ dissolves in seawater, it reacts with water to form carbonic acid:
CO₂ + H₂O ⇌ H₂CO₃
This increases the concentration of H⁺ ions in the ocean, lowering its pH. The decrease in pH has detrimental effects on marine organisms, particularly those with calcium carbonate shells and skeletons, like corals and shellfish. The increased acidity makes it more difficult for these organisms to build and maintain their shells, threatening their survival and impacting the entire marine ecosystem.
Industrial Applications of Carbonic Acid
While not as commonly used as strong acids in industrial processes, carbonic acid finds application in specific areas. For instance, it’s utilized in:
- Carbonated beverages: The fizzy sensation in soda is due to the dissolved carbon dioxide, which forms carbonic acid in the beverage.
- Food processing: Carbonic acid can act as a leavening agent in some food products.
- Cleaning and sanitation: In some instances, it can be used as a mild cleaning agent.
Comparing Carbonic Acid to Other Weak Acids
It's helpful to compare carbonic acid to other common weak acids to better understand its relative strength. Acetic acid (CH₃COOH), found in vinegar, has a Ka value of approximately 1.8 x 10⁻⁵. This is significantly larger than the Ka₁ of carbonic acid, indicating that acetic acid is a stronger acid than carbonic acid. Similarly, other weak acids like lactic acid and formic acid also exhibit higher Ka values than carbonic acid.
Conclusion: The Significance of a Weak Acid
Carbonic acid, despite its weakness as an acid, plays a crucial and multifaceted role in various natural and industrial processes. Its incomplete dissociation, leading to a buffering capacity and equilibrium reactions, is essential for maintaining pH homeostasis in biological systems and influencing ocean chemistry. Understanding its properties, particularly its relative weakness compared to other acids, is vital for comprehending its impact on diverse environments and systems. Further research continues to uncover the intricacies of its behavior and its broader significance in the natural world. The ongoing study of carbonic acid and its effects highlights the importance of understanding the chemical properties of weak acids in various contexts, from the microscopic level of biological processes to the global scale of environmental change. The ongoing investigation into carbonic acid's behavior in different conditions promises to yield further insights into its multifaceted role in the natural world and its influence on various systems.
Latest Posts
Latest Posts
-
Diagram Of An Animal And Plant Cell
Mar 29, 2025
-
What Is The Difference Between Environment And Ecosystem
Mar 29, 2025
-
How To Find Average Speed With Two Speeds
Mar 29, 2025
-
A Subset Of A Population Is Called A
Mar 29, 2025
-
The Ultimate Source Of Energy For Most Organisms Is
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Carbonic Acid Strong Or Weak Acid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
