Write The Electron Configuration For A Neutral Atom Of Tin
Juapaving
Mar 21, 2025 · 6 min read
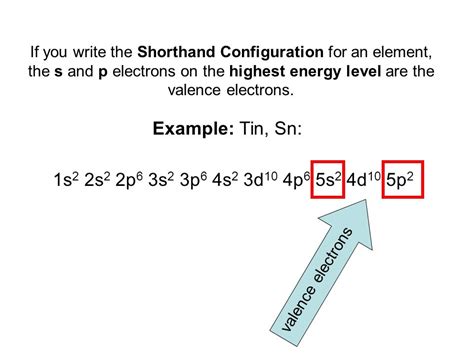
Table of Contents
Unveiling the Electronic Structure of Tin: A Deep Dive into its Electron Configuration
Tin (Sn), a fascinating element residing in Group 14 of the periodic table, boasts a rich history and a diverse range of applications. From ancient bronze alloys to modern-day electronics, tin's unique properties are inextricably linked to its electronic structure. This article delves into the intricacies of determining the electron configuration of a neutral tin atom, exploring the underlying principles and providing a comprehensive understanding of its atomic arrangement.
Understanding Electron Configurations
Before we embark on the journey of configuring tin's electrons, let's refresh our understanding of electron configurations. An electron configuration is a symbolic representation of the arrangement of electrons in the various energy levels and sublevels within an atom. It follows the Aufbau principle, which states that electrons fill the lowest energy levels first. This principle is governed by the Pauli exclusion principle, which dictates that no two electrons in an atom can have the same four quantum numbers (n, l, ml, and ms). Finally, Hund's rule dictates that electrons will individually occupy each orbital within a subshell before doubling up in any one orbital.
Understanding these principles is crucial for accurately predicting and interpreting electron configurations. Each electron occupies a specific orbital, characterized by its principal quantum number (n), which determines the energy level, and its azimuthal quantum number (l), which defines the subshell (s, p, d, or f).
Determining the Electron Configuration of Tin (Sn)
Tin has an atomic number of 50, meaning a neutral tin atom possesses 50 electrons. To determine its electron configuration, we systematically fill the orbitals according to the Aufbau principle and Hund's rule, keeping the Pauli exclusion principle in mind.
The order of filling orbitals is typically represented as: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p... and so on. However, remember that this is a simplified representation and slight deviations can occur due to subtle energy level interactions.
Let's now build the electron configuration for tin (Sn):
- 1s²: The first energy level (n=1) contains the 1s subshell, which can hold a maximum of two electrons.
- 2s² 2p⁶: The second energy level (n=2) has a 2s subshell (two electrons) and a 2p subshell (six electrons).
- 3s² 3p⁶: The third energy level (n=3) also follows the same pattern with a 3s and a 3p subshell.
- 4s² 3d¹⁰ 4p⁶: The fourth energy level is where things get slightly more complex. It includes the 4s, 3d, and 4p subshells. Note that the 3d subshell fills after the 4s subshell.
- 5s² 4d¹⁰ 5p²: Finally, we reach the fifth energy level, which houses the valence electrons in tin. It contains the 5s, 4d, and 5p subshells. Tin's valence electrons reside in the 5s and 5p subshells.
Therefore, the complete electron configuration for a neutral tin atom is: 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶ 5s² 4d¹⁰ 5p².
This can also be written in a condensed or shorthand notation using the noble gas configuration: [Kr] 5s² 4d¹⁰ 5p². Here, [Kr] represents the electron configuration of krypton (1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶), which is the noble gas preceding tin in the periodic table.
Understanding the Significance of Tin's Electron Configuration
Tin's electron configuration provides valuable insights into its chemical and physical properties. The presence of four valence electrons (two in the 5s and two in the 5p subshells) explains its ability to form a variety of compounds, exhibiting oxidation states of +2 and +4.
The +2 oxidation state arises from the loss of the two 5p electrons, while the +4 oxidation state involves the loss of all four valence electrons (5s² and 5p²). This versatility in oxidation states contributes significantly to tin's wide array of applications.
Furthermore, the completely filled 4d subshell contributes to the relatively high density and metallic character of tin. The interaction between the valence electrons and the inner shell electrons influences many physical properties, such as melting point and electrical conductivity.
Tin's Allotropes and Their Relationship to Electron Configuration
Tin exhibits allotropy, meaning it exists in different structural forms. The most common allotropes are:
- α-tin (grey tin): This is a brittle, non-metallic form stable below 13.2 °C. Its structure is diamond-like, suggesting a significant influence from the underlying electronic structure.
- β-tin (white tin): This is the metallic form stable above 13.2 °C and is the most commonly encountered form of tin. Its tetragonal structure reflects the delocalized nature of its valence electrons.
The transformation between these allotropes is influenced by temperature and is associated with changes in the electron distribution and bonding within the crystal lattice. The specific arrangement of electrons dictates the stability and properties of each allotrope.
Applications of Tin and its Correlation with Electron Configuration
The electron configuration of tin underpins its wide range of applications across various industries:
- Soldering: Tin's low melting point and excellent wetting properties, stemming from its electronic structure, make it indispensable in soldering electronics.
- Coatings: Tin coatings offer corrosion resistance to metals, a property closely linked to its ability to form stable oxides.
- Alloys: Tin alloys, such as bronze (copper-tin alloy) and pewter (tin-antimony-copper alloy), have been utilized for centuries due to their unique mechanical properties. The electronic interactions between tin and other constituent metals result in enhanced material properties.
- Organotin compounds: These compounds find extensive applications as biocides, stabilizers in plastics, and catalysts. The ability of tin to form bonds with carbon atoms, related to its electron configuration, is crucial in these applications.
Advanced Concepts and Further Exploration
While the basic electron configuration provides a foundational understanding, a more detailed analysis requires exploring concepts like:
- Relativistic effects: For heavier elements like tin, relativistic effects become significant, influencing the energy levels and orbital sizes.
- Effective nuclear charge: This concept accounts for the shielding effect of inner electrons on the valence electrons, providing a more accurate picture of the electron-nucleus interaction.
- Computational chemistry: Advanced computational methods can be used to calculate more accurate electron densities and energy levels, offering deeper insights into the electronic structure.
Understanding these advanced concepts enhances the accuracy of our understanding of tin's properties and behavior.
Conclusion
The electron configuration of tin, [Kr] 5s² 4d¹⁰ 5p², is the key to unlocking its fascinating properties and diverse applications. By understanding the fundamental principles of electron configuration and the significance of its valence electrons, we gain valuable insights into tin's behavior in various chemical and physical contexts. From its allotropic transformations to its role in diverse materials and applications, the electronic structure of tin lies at the heart of its unique characteristics and industrial importance. This detailed exploration provides a comprehensive understanding of this important element and its place in the world of chemistry and materials science. Further research and exploration into the intricacies of its electronic structure will undoubtedly reveal even more about its potential and applications in the future.
Latest Posts
Latest Posts
-
Which Pair Of Triangle Is Congruent By Asa
Mar 22, 2025
-
What Forms When Two Atoms Combine
Mar 22, 2025
-
Who Performed Religious Rituals In Early Hinduism
Mar 22, 2025
-
What Is Pseudo First Order Reaction
Mar 22, 2025
-
What Are 2 Ways In Which Mixtures Differ From Compounds
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about Write The Electron Configuration For A Neutral Atom Of Tin . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
