What Are 2 Ways In Which Mixtures Differ From Compounds
Juapaving
Mar 22, 2025 · 6 min read
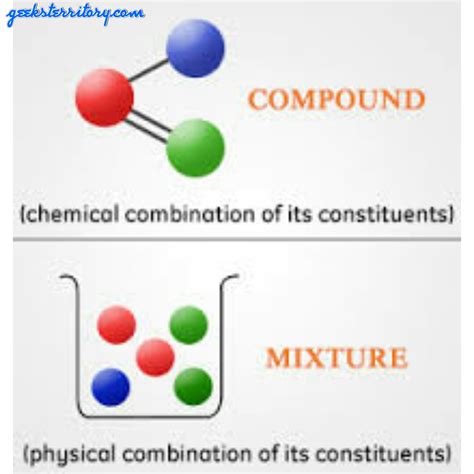
Table of Contents
What Are 2 Ways in Which Mixtures Differ From Compounds?
Chemistry, at its core, is the study of matter and its transformations. Understanding the fundamental differences between mixtures and compounds is crucial to grasping many chemical concepts. While both mixtures and compounds consist of two or more substances, their inherent nature and properties differ significantly. This article delves into two key distinctions between mixtures and compounds: the method of separation and the composition's consistency. Understanding these differences provides a solid foundation for further exploration of chemical principles.
1. Method of Separation: A Defining Characteristic
One of the most fundamental ways to differentiate between mixtures and compounds lies in how their constituent parts can be separated. This difference stems from the nature of the bonds holding the substances together.
Mixtures: Separable by Physical Methods
Mixtures are combinations of two or more substances that are physically combined. This means the individual components retain their original chemical identities and properties. Crucially, the components of a mixture are not chemically bonded to each other. This lack of chemical bonds allows for separation using relatively simple physical methods, which don't alter the chemical nature of the components.
Examples of physical separation techniques include:
-
Filtration: This separates solids from liquids based on particle size. Think of using a coffee filter to separate coffee grounds from brewed coffee. The coffee grounds (solid) are trapped by the filter, while the coffee (liquid) passes through.
-
Evaporation: This separates dissolved solids from liquids by allowing the liquid to evaporate, leaving behind the solid residue. Sea salt is obtained from seawater through evaporation – the water evaporates, leaving behind the salt crystals.
-
Distillation: This technique utilizes differences in boiling points to separate liquids. Crude oil is refined through fractional distillation, separating it into various components like gasoline, kerosene, and diesel fuel, each boiling at a different temperature.
-
Chromatography: This technique separates substances based on their differing affinities for a stationary and a mobile phase. It’s frequently used in analytical chemistry to separate complex mixtures into their individual components, for example, separating pigments in ink.
-
Magnetic Separation: This method exploits the magnetic properties of certain substances to separate them from non-magnetic ones. For instance, iron filings can be easily separated from sand using a magnet.
These physical methods are effective because they don't involve breaking or forming chemical bonds. The substances simply separate based on their physical properties.
Compounds: Separable Only by Chemical Methods
In contrast to mixtures, compounds are formed when two or more elements combine chemically, resulting in a new substance with different properties from its constituent elements. This chemical combination involves the formation of chemical bonds, typically ionic or covalent bonds. The constituent elements are no longer identifiable as they were before the chemical reaction. Consequently, separating a compound into its constituent elements requires chemical methods, involving processes that break the chemical bonds.
Examples of chemical separation techniques include:
-
Electrolysis: This uses an electric current to decompose a compound into its elements. For example, the electrolysis of water (H₂O) breaks it down into hydrogen (H₂) and oxygen (O₂).
-
Chemical Reactions: Many compounds can be decomposed into simpler substances through controlled chemical reactions. For instance, heating mercury(II) oxide (HgO) produces mercury (Hg) and oxygen (O₂).
-
Thermal Decomposition: This involves heating a compound until it breaks down into simpler substances. Many carbonates, for example, decompose upon heating to produce metal oxides and carbon dioxide.
These chemical methods are necessary because they target and break the chemical bonds holding the compound together. The resulting elements or simpler compounds have distinctly different properties than the original compound.
In summary: Mixtures are separable by physical methods because their components are not chemically bonded, while compounds require chemical methods for separation due to the presence of chemical bonds. This fundamental difference is a cornerstone of understanding the distinction between mixtures and compounds.
2. Composition's Consistency: A Matter of Uniformity
Another crucial way to differentiate mixtures from compounds is the consistency of their composition. This relates to the uniformity of the mixture or the fixed ratio of elements in a compound.
Mixtures: Variable Composition
Mixtures can have a variable composition. This means the ratio of the components can vary widely without changing the fundamental nature of the mixture. For example, you can make a saltwater solution with a high concentration of salt or a low concentration of salt, and it remains a saltwater solution. The proportion of salt to water can be adjusted without fundamentally altering the identity of the mixture. Similarly, a sand and water mixture can have varying amounts of sand and water, still maintaining its overall identity. This variability in composition is a key feature of mixtures. They are heterogeneous (non-uniform) or homogeneous (uniform) at the macroscopic level, but on a microscopic level, they are inherently non-uniform.
This variable composition is a direct consequence of the lack of chemical bonding between the components. Each component retains its individuality, and their proportions can be altered without affecting their chemical identity within the mixture.
Compounds: Fixed Composition (Law of Definite Proportions)
Compounds, however, always have a fixed composition, adhering to the Law of Definite Proportions. This law states that a given chemical compound always contains its constituent elements in a fixed ratio (by mass). For instance, water (H₂O) always contains two hydrogen atoms for every one oxygen atom. This ratio is consistent irrespective of the source of the water (rainwater, seawater, etc.). Similarly, sodium chloride (NaCl) always contains one sodium atom for every one chlorine atom. This fixed composition arises from the nature of chemical bonds – the atoms are specifically linked together in a precise arrangement dictated by the chemical formula.
The fixed composition of compounds is a reflection of the strong chemical bonds holding the atoms together. Changing the ratio of elements would result in a different compound entirely, not merely a different proportion within the same compound. This fixed ratio defines the compound's unique chemical identity and properties.
In summary: Mixtures demonstrate variable composition, with the ratio of components being readily adjustable. Compounds, in contrast, exhibit a fixed composition, dictated by the Law of Definite Proportions and the chemical bonds present.
Conclusion: A Clear Distinction
The two key differences highlighted – the method of separation and the consistency of composition – provide a robust framework for differentiating between mixtures and compounds. Mixtures are separable by physical methods due to the lack of chemical bonds between their components, and their composition is variable. Conversely, compounds require chemical methods for separation because of the presence of chemical bonds, and they always possess a fixed composition according to the Law of Definite Proportions. Understanding these fundamental distinctions is essential for comprehending the basic principles of chemistry and for analyzing the behaviour of matter. It underpins further studies in stoichiometry, chemical reactions, and material science. By grasping these core concepts, you lay a solid foundation for a deeper understanding of the world around you, at the molecular level.
Latest Posts
Latest Posts
-
The Numerical Factor Of A Term
Mar 24, 2025
-
What Is The Venation Of A Leaf
Mar 24, 2025
-
What Is The Lcm Of 72 And 120
Mar 24, 2025
-
What Are Two Ways Mixtures Differ From Compounds
Mar 24, 2025
-
Which Is Not A Measure Of Dispersion
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about What Are 2 Ways In Which Mixtures Differ From Compounds . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
