What Is Pseudo First Order Reaction
Juapaving
Mar 22, 2025 · 6 min read
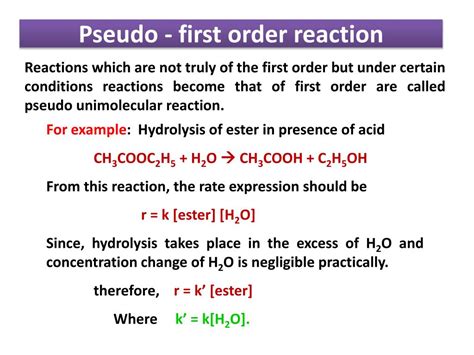
Table of Contents
What is a Pseudo First-Order Reaction? A Comprehensive Guide
Understanding reaction kinetics is crucial in chemistry, and within this field, pseudo first-order reactions hold a significant place. While seemingly complex, grasping the concept of a pseudo first-order reaction opens doors to a deeper understanding of reaction mechanisms and rate determination. This comprehensive guide delves into the intricacies of pseudo first-order reactions, exploring their definition, characteristics, examples, and practical applications.
Defining Pseudo First-Order Reactions
A pseudo first-order reaction is a chemical reaction that appears to follow first-order kinetics even though it's actually a higher-order reaction. This apparent simplification occurs when the concentration of one or more reactants is significantly greater than the concentration of the other reactant(s). The higher concentration reactant(s) remains essentially constant throughout the reaction, while the concentration of the lower concentration reactant(s) changes significantly. This effectively makes the rate of the reaction dependent only on the concentration of the lower concentration reactant, mimicking a first-order reaction.
It's crucial to understand that a pseudo first-order reaction is not truly a first-order reaction; it merely behaves like one under specific conditions. The true order of the reaction remains unchanged, but the observed kinetics are simplified due to the overwhelming excess of one or more reactants.
Key Characteristics of Pseudo First-Order Reactions
- Apparent First-Order Kinetics: The rate law appears to be first-order, with the rate depending only on the concentration of the reactant present in lower concentration.
- Concentration Dependence: The rate is directly proportional to the concentration of the limiting reactant. Doubling the concentration of the limiting reactant doubles the rate of the reaction.
- Excess Reactant: One or more reactants are present in a significantly large excess compared to the other reactant(s). This excess ensures that the concentration of the abundant reactant remains virtually constant during the course of the reaction.
- Simplified Rate Law: The complex rate law of the higher-order reaction simplifies to a first-order rate law because the concentration term(s) of the reactant(s) in excess are considered constant and incorporated into the rate constant.
- Rate Constant Dependence: The observed rate constant (k<sub>obs</sub>) is dependent on the concentration of the reactant(s) in excess.
Understanding the Rate Law Transformation
To illustrate the transformation of a higher-order reaction into a pseudo first-order reaction, let's consider a general second-order reaction:
A + B → Products
The actual rate law for this reaction is:
Rate = k[A][B]
where:
- k is the true rate constant
- [A] and [B] are the concentrations of reactants A and B, respectively.
Now, let's assume that the concentration of reactant B is much greater than the concentration of reactant A ([B] >> [A]). In this scenario, the concentration of B remains essentially constant throughout the reaction. We can then incorporate [B] into the rate constant, creating a new observed rate constant (k<sub>obs</sub>):
k<sub>obs</sub> = k[B]
The rate law now simplifies to:
Rate = k<sub>obs</sub>[A]
This equation resembles a first-order rate law, where the rate is solely dependent on the concentration of A. Therefore, even though the actual reaction is second-order, it behaves as a pseudo first-order reaction under these specific conditions.
Examples of Pseudo First-Order Reactions
Many chemical reactions exhibit pseudo first-order behavior under appropriate conditions. Here are some prominent examples:
1. Hydrolysis of Esters
The hydrolysis of esters in the presence of excess water is a classic example. The reaction is second-order overall but becomes pseudo first-order when water is present in large excess. The rate then depends only on the ester concentration.
RCOOR' + H₂O → RCOOH + R'OH
2. Inversion of Sucrose
The acid-catalyzed inversion of sucrose is another common example. The reaction is also second-order but exhibits pseudo first-order kinetics when a large excess of acid is used. The rate is then solely determined by the sucrose concentration.
C₁₂H₂₂O₁₁ + H₂O → C₆H₁₂O₆ + C₆H₁₂O₆
(Sucrose + Water → Glucose + Fructose)
3. Enzyme Catalysis
Many enzyme-catalyzed reactions exhibit pseudo first-order kinetics when the substrate concentration is much lower than the enzyme concentration. The enzyme concentration is effectively constant, making the reaction rate dependent primarily on the substrate concentration.
4. Decomposition of Diazonium Salts
The decomposition of certain diazonium salts in aqueous solution follows pseudo first-order kinetics under conditions of excess water.
Determining the Order and Rate Constant
Experimentally determining the order and rate constant for a pseudo first-order reaction requires careful consideration. The simplified rate law, while useful for kinetic analysis under specific conditions, does not reveal the true order of the reaction.
To determine the true order, you need to perform experiments with varying concentrations of both reactants. This allows for the determination of the overall reaction order and the true rate constant. However, the pseudo first-order approach is valuable for simplifying the analysis under specific concentration conditions, making it easier to calculate the observed rate constant, k<sub>obs</sub>.
Typically, graphical methods are employed using the integrated rate law for first-order reactions:
ln([A]<sub>t</sub>) = -k<sub>obs</sub>t + ln([A]<sub>0</sub>)
where:
- [A]<sub>t</sub> is the concentration of A at time t
- [A]<sub>0</sub> is the initial concentration of A
- k<sub>obs</sub> is the observed rate constant
- t is the time
Plotting ln([A]<sub>t</sub>) against time (t) yields a straight line with a slope of -k<sub>obs</sub>. This graphical method allows for easy determination of the observed rate constant.
Practical Applications of Pseudo First-Order Reactions
Pseudo first-order reactions find extensive applications in various fields:
- Pharmacokinetics: Studying drug metabolism and elimination often involves pseudo first-order kinetics because the drug concentration is typically much lower than the concentration of metabolizing enzymes.
- Environmental Chemistry: Analyzing the degradation of pollutants in the environment, where the pollutant concentration is significantly less than the concentration of degrading agents (e.g., microorganisms).
- Chemical Engineering: Designing and optimizing chemical reactors where one reactant is present in a large excess to simplify the reaction kinetics.
- Analytical Chemistry: Determining reaction rates and mechanisms, particularly when dealing with complex reactions involving multiple reactants.
Distinguishing Pseudo First-Order from True First-Order Reactions
It's crucial to understand the distinction between a true first-order reaction and a pseudo first-order reaction. A true first-order reaction inherently depends on the concentration of only one reactant, irrespective of the concentrations of other reactants. The rate constant for a true first-order reaction is independent of the concentrations of any other species in the reaction. Conversely, the observed rate constant (k<sub>obs</sub>) for a pseudo first-order reaction is directly influenced by the concentration of the reactant(s) in excess.
Conclusion
Pseudo first-order reactions, while not truly first-order, provide a simplified approach to analyzing reaction kinetics when one or more reactants are present in vast excess. This simplification makes rate determination and mechanism elucidation more manageable. Understanding this concept is critical for researchers and students alike, enabling a deeper comprehension of reaction mechanisms and their practical applications in various scientific disciplines. By recognizing the conditions under which a reaction behaves pseudo first-order, we can accurately analyze reaction kinetics and make informed predictions about reaction rates and outcomes. The techniques and principles discussed here are fundamental to various aspects of chemistry and related fields, highlighting the importance of understanding this specific type of reaction kinetics.
Latest Posts
Latest Posts
-
Which Type Of Plastids Store Food
Mar 24, 2025
-
The Function Of A Lacteal Is To Absorb
Mar 24, 2025
-
If Meiosis Did Not Occur In Sexually Reproducing Organisms
Mar 24, 2025
-
What Is The Lateral Surface Area Of A Rectangular Prism
Mar 24, 2025
-
Acids Turn Litmus Paper What Color
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about What Is Pseudo First Order Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
