What Forms When Two Atoms Combine
Juapaving
Mar 22, 2025 · 6 min read
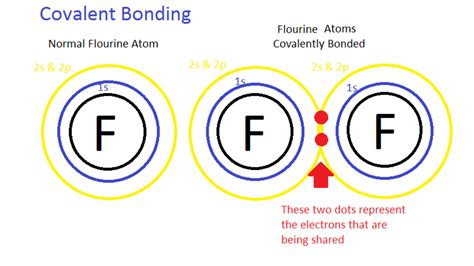
Table of Contents
What Forms When Two Atoms Combine? Exploring Chemical Bonds and Molecular Structures
When two atoms combine, they form a chemical bond, creating a new structure called a molecule or, in the case of large assemblies, a compound. This process is fundamental to chemistry and underpins the existence of all matter as we know it. Understanding how and why atoms bond is key to comprehending the properties of substances and predicting their behavior. This comprehensive guide delves into the intricacies of atomic interactions, exploring the various types of chemical bonds and the resulting molecular structures.
The Driving Force: Achieving Stability
Atoms combine to achieve a more stable electron configuration. Atoms are inherently unstable when their outermost electron shell, also known as the valence shell, is incomplete. According to the octet rule (with exceptions for elements like hydrogen and lithium), atoms strive to have eight electrons in their valence shell to achieve a stable, noble gas-like configuration. This drive for stability is the primary force that dictates chemical bonding.
Energy Considerations
The formation of a chemical bond is always accompanied by a release of energy. This energy release signifies the increased stability of the bonded atoms compared to their isolated states. The energy required to break a chemical bond is known as the bond dissociation energy, a measure of the bond's strength. The greater the bond dissociation energy, the stronger and more stable the bond.
Types of Chemical Bonds
Several types of chemical bonds exist, each characterized by how atoms share or transfer electrons:
1. Ionic Bonds: The Transfer of Electrons
Ionic bonds form when one atom transfers one or more electrons to another atom. This transfer creates two oppositely charged ions: a positively charged cation (the atom that lost electrons) and a negatively charged anion (the atom that gained electrons). The electrostatic attraction between these oppositely charged ions constitutes the ionic bond.
Example: Sodium (Na) readily loses one electron to achieve a stable configuration, becoming a Na⁺ cation. Chlorine (Cl) readily gains one electron to achieve a stable configuration, becoming a Cl⁻ anion. The electrostatic attraction between Na⁺ and Cl⁻ forms the ionic bond in sodium chloride (NaCl), or common table salt.
Characteristics of Ionic Compounds:
- High melting and boiling points due to the strong electrostatic forces between ions.
- Crystalline structure, reflecting the ordered arrangement of ions in a lattice.
- Generally soluble in polar solvents like water.
- Conduct electricity when molten or dissolved in water due to the mobility of ions.
2. Covalent Bonds: The Sharing of Electrons
Covalent bonds form when two or more atoms share one or more pairs of electrons to achieve a stable electron configuration. This sharing occurs when atoms have similar electronegativities, meaning they have a similar attraction for electrons.
Examples: The bond in a hydrogen molecule (H₂) involves the sharing of one electron pair between two hydrogen atoms. In methane (CH₄), carbon shares four electron pairs with four hydrogen atoms.
Types of Covalent Bonds:
- Nonpolar Covalent Bonds: Electrons are shared equally between atoms of the same element or atoms with very similar electronegativities. Examples include H₂, O₂, and Cl₂.
- Polar Covalent Bonds: Electrons are shared unequally between atoms with different electronegativities. This creates a dipole moment, with one atom having a slightly positive charge (δ+) and the other a slightly negative charge (δ−). Examples include H₂O and HCl.
Characteristics of Covalent Compounds:
- Lower melting and boiling points compared to ionic compounds.
- Can exist as solids, liquids, or gases at room temperature.
- Generally less soluble in water than ionic compounds.
- Poor conductors of electricity in their solid state but may conduct electricity when dissolved in certain solvents or in the gaseous phase.
3. Metallic Bonds: A Sea of Electrons
Metallic bonds occur in metals, where valence electrons are delocalized and form a "sea" of electrons surrounding positively charged metal ions. These delocalized electrons are not associated with any particular atom and are free to move throughout the metal structure.
Characteristics of Metallic Compounds:
- High electrical and thermal conductivity due to the mobility of delocalized electrons.
- Malleability and ductility, meaning they can be easily shaped and drawn into wires.
- Lustrous appearance due to the interaction of light with the delocalized electrons.
4. Hydrogen Bonds: Special Intermolecular Forces
While not a true chemical bond in the same way as ionic or covalent bonds, hydrogen bonds are a type of strong intermolecular force that occurs between a hydrogen atom bonded to a highly electronegative atom (like oxygen, nitrogen, or fluorine) and another electronegative atom in a different molecule. These forces play a crucial role in the properties of many biological molecules like water and proteins.
Molecular Geometry and Shape
The arrangement of atoms in a molecule, known as its molecular geometry or shape, significantly influences its physical and chemical properties. The shape is determined by the number of bonding pairs and lone pairs of electrons around the central atom, and it can be predicted using models like the Valence Shell Electron Pair Repulsion (VSEPR) theory.
Some common molecular shapes include:
- Linear: Atoms are arranged in a straight line (e.g., CO₂).
- Bent: Atoms are arranged in a V-shape (e.g., H₂O).
- Trigonal planar: Atoms are arranged in a flat triangle (e.g., BF₃).
- Tetrahedral: Atoms are arranged in a three-dimensional tetrahedron (e.g., CH₄).
- Octahedral: Atoms are arranged in an octahedron (e.g., SF₆)
The Role of Electronegativity
Electronegativity is a crucial factor in determining the type of bond formed between atoms. It refers to the ability of an atom to attract electrons in a chemical bond. A large difference in electronegativity between two atoms leads to an ionic bond, while a small difference leads to a covalent bond. The electronegativity difference can also be used to predict the polarity of a covalent bond.
Beyond Simple Bonds: Complex Structures
Many molecules and compounds exhibit more complex bonding structures than simple single bonds. These include:
- Double bonds: Two pairs of electrons are shared between two atoms (e.g., O₂).
- Triple bonds: Three pairs of electrons are shared between two atoms (e.g., N₂).
- Coordinate covalent bonds (dative bonds): Both electrons in a shared pair are donated by one atom.
- Resonance structures: Molecules with delocalized electrons, where the bonding electrons are spread over multiple atoms (e.g., benzene).
- Polymers: Large molecules consisting of repeating structural units (e.g., polyethylene).
- Metallic Clusters and Alloys: Complex combinations of metallic elements with intricate bonding arrangements.
Conclusion
The combination of two atoms, a seemingly simple process, results in a diverse array of molecules and compounds with unique properties and applications. The type of bond formed, determined primarily by the electronegativity difference between atoms and their electronic configurations, dictates the behavior of the resultant substance. From the simple ionic bond in table salt to the complex covalent networks in biological molecules and the delocalized electron sea in metals, chemical bonding underlies the fundamental structure and properties of the physical world around us. Understanding chemical bonds is essential for advancements in materials science, pharmaceuticals, and countless other scientific fields. Further exploration of this fascinating topic reveals an even more intricate and beautiful picture of the building blocks of matter.
Latest Posts
Latest Posts
-
Two Pairs Of Opposite Sides Are Parallel
Mar 24, 2025
-
What Is The Factor Of 83
Mar 24, 2025
-
The Numerical Factor Of A Term
Mar 24, 2025
-
What Is The Venation Of A Leaf
Mar 24, 2025
-
What Is The Lcm Of 72 And 120
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about What Forms When Two Atoms Combine . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
