Who Was The First To Propose The Existence Of Atoms
Juapaving
Mar 07, 2025 · 6 min read
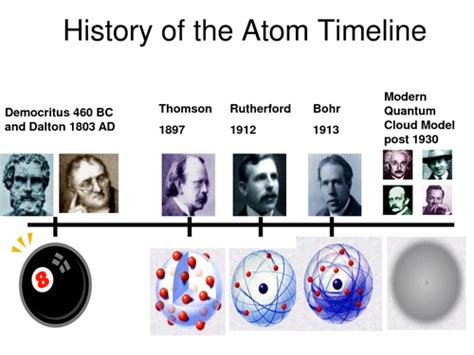
Table of Contents
The Enduring Quest: Who First Proposed the Existence of Atoms?
The concept of the atom, the fundamental building block of matter, is a cornerstone of modern science. But the journey to understanding its existence has been a long and winding one, spanning millennia and involving numerous thinkers from diverse cultures. Pinpointing the very first person to propose the existence of atoms is a challenging task, as the idea evolved gradually, often expressed implicitly rather than explicitly, and frequently intertwined with philosophical rather than scientific inquiry. However, by exploring the historical context and examining key figures, we can illuminate the path that led to our current atomic understanding.
Ancient Greece: The Dawn of Atomic Thought
While the precise origins remain shrouded in mystery, ancient Greece stands out as a crucial birthplace of atomic ideas. Several pre-Socratic philosophers, grappling with the fundamental nature of reality, independently developed concepts that foreshadowed the atom.
Democritus and Leucippus: The Atomos
The most commonly credited proponents of atomism in ancient Greece are Democritus (c. 460 – c. 370 BC) and his teacher, Leucippus (5th century BC). Their theory, though lacking the experimental rigor of modern science, stands as a remarkable intellectual achievement. They proposed that all matter is composed of indivisible, indestructible particles called atomos (ἄτομος), meaning "uncuttable" or "indivisible."
-
The Nature of Atomos: Democritus described these atomos as eternally existing, solid, and homogenous particles differing only in shape, size, and arrangement. These variations, according to their theory, explained the diversity of substances observed in the world. The properties of matter were determined by the types and arrangement of these fundamental particles.
-
The Void: A crucial aspect of their theory was the acknowledgment of empty space, or void, between the atomos. This concept challenged the prevailing belief that space was entirely filled with matter. This void allowed for motion and interaction between the atoms, a crucial step in conceptualizing a dynamic universe.
-
Limitations of their Theory: It's crucial to acknowledge that the atomic theory of Democritus and Leucippus was purely philosophical. They lacked the tools and methods to experimentally verify their claims. Their arguments relied heavily on logical deduction and reasoned speculation rather than empirical observation.
Beyond Greece: Other Early Contributions
While Democritus and Leucippus are most frequently associated with the atomic concept in the West, it's important to note that similar ideas might have arisen independently in other cultures. Limited evidence suggests that certain philosophical schools in India and possibly China explored related concepts of fundamental indivisible particles. However, the lack of detailed records makes it difficult to assess the degree of similarity or independent development.
The Long Interlude: From Antiquity to the Scientific Revolution
After the flourishing of Greek philosophy, the atom concept largely faded from mainstream scientific thought for centuries. The dominance of Aristotelian physics, which emphasized the continuous nature of matter, overshadowed the atomic view. The emphasis shifted towards qualitative descriptions of natural phenomena rather than the quantitative analysis needed to understand atomic behavior.
The prevailing scholastic tradition of the Middle Ages and the Renaissance did little to revive the atomist perspective. While some thinkers engaged with the ideas of ancient Greece, the focus remained on theological and metaphysical interpretations rather than empirical investigation.
The Scientific Revolution and the Revival of Atomism
The scientific revolution of the 16th and 17th centuries marked a turning point. The emphasis on observation, experimentation, and mathematical reasoning paved the way for the eventual resurgence of atomic ideas. While not explicitly proposing atomic theory, several key figures laid the groundwork for its eventual acceptance.
Isaac Newton and the Corpuscularian Philosophy:
Sir Isaac Newton (1643-1727), though primarily known for his laws of motion and universal gravitation, held a corpuscularian view of matter. He believed that matter was composed of tiny particles, though not necessarily indivisible atoms in the sense of Democritus. His work, particularly his optical experiments, demonstrated the particulate nature of light, influencing subsequent thinking about the nature of matter.
Robert Boyle and the Chemical Revolution:
Robert Boyle (1627-1691) played a critical role in establishing chemistry as a distinct scientific discipline. His experiments, especially on gases, helped to refine the understanding of chemical reactions and the behavior of matter. Though not explicitly advocating atomism, Boyle's meticulous observations and quantitative approach created a fertile ground for its later acceptance.
The 18th and 19th Centuries: Towards a Modern Atomic Theory
The 18th and 19th centuries witnessed a growing convergence of scientific evidence supporting the existence of atoms. Several scientists contributed to this gradual shift:
Antoine Lavoisier and the Law of Conservation of Mass:
Antoine Lavoisier (1743-1794), considered the "father of modern chemistry," emphasized the importance of precise measurement and quantitative analysis. His experiments established the law of conservation of mass, stating that matter cannot be created or destroyed in a chemical reaction. This crucial principle provided further support for the idea of discrete, indivisible particles.
John Dalton and the Atomic Theory:
John Dalton (1766-1844) is widely regarded as the father of modern atomic theory. Building upon the work of his predecessors, Dalton formulated a comprehensive atomic theory that included several postulates:
- All matter is composed of atoms, which are indivisible and indestructible particles. This directly echoes the ideas of Democritus and Leucippus.
- All atoms of a given element are identical in mass and properties. This implied a fundamental distinction between different elements.
- Compounds are formed by a combination of two or more different kinds of atoms. This provided a mechanistic explanation for the formation of chemical compounds.
- A chemical reaction is a rearrangement of atoms. This explained why mass is conserved during chemical reactions.
Dalton's atomic theory, unlike the earlier philosophical speculations, was grounded in experimental evidence and provided a framework for understanding chemical reactions and the composition of matter. Although some of Dalton's postulates were later refined or revised (e.g., atoms are not completely indivisible), his theory represents a landmark achievement in the development of atomic theory.
The 20th Century and Beyond: The Subatomic World
The 20th century saw a revolution in our understanding of the atom with the discovery of subatomic particles – electrons, protons, and neutrons. This revealed that atoms, while fundamental building blocks, are not themselves indivisible. The development of quantum mechanics further refined our understanding of atomic structure and behavior, revealing the complex and probabilistic nature of the atomic realm.
Conclusion: A Collaborative Journey
In conclusion, while Democritus and Leucippus are often credited as the first to propose the existence of atoms, it's essential to view their contribution within a broader historical context. Their ideas were largely philosophical, lacking the experimental validation that characterized later developments. The evolution of atomic theory was a collaborative and iterative process spanning millennia, involving numerous thinkers from different cultures and scientific disciplines. From the philosophical speculations of ancient Greece to the experimental breakthroughs of the 19th and 20th centuries, the quest to understand the atom has been a continuous journey of discovery, shaping our understanding of the universe and its fundamental constituents. The legacy of Democritus and Leucippus remains, however, as the foundational thinkers who planted the seed of an idea that would eventually blossom into one of science's most profound discoveries.
Latest Posts
Latest Posts
-
How Many Valence Electrons In Br
Mar 09, 2025
-
Identify The Following Physical Quantities As Scalars Or Vectors
Mar 09, 2025
-
How To Find A Coordination Number
Mar 09, 2025
-
Is 97 A Prime Number Or A Composite Number
Mar 09, 2025
-
Give One Reason Mendel Chose Pea Plants For His Experiment
Mar 09, 2025
Related Post
Thank you for visiting our website which covers about Who Was The First To Propose The Existence Of Atoms . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
