How To Find A Coordination Number
Juapaving
Mar 09, 2025 · 6 min read
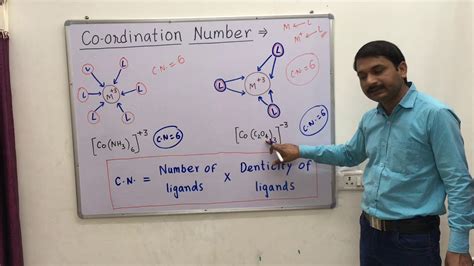
Table of Contents
How to Find the Coordination Number: A Comprehensive Guide
Determining the coordination number of a central atom or ion within a compound is crucial in understanding its structure, properties, and reactivity. The coordination number (CN) simply represents the number of atoms, ions, or molecules directly bonded to the central atom. This seemingly straightforward concept can become surprisingly nuanced, demanding careful consideration of various factors. This comprehensive guide will explore the different approaches to determining coordination numbers, addressing both simple and complex scenarios.
Understanding the Fundamentals: What is a Coordination Number?
Before delving into the methods, let's solidify our understanding. The coordination number reflects the immediate environment surrounding a central atom. It's not about the total number of atoms in a molecule, but specifically those directly connected to the central atom through a chemical bond – be it ionic, covalent, or metallic.
Consider a simple example: in [Cu(H₂O)₆]²⁺, the copper(II) ion (Cu²⁺) is surrounded by six water molecules. Therefore, the coordination number of copper in this complex ion is 6. Each water molecule forms a coordinate bond with the central copper ion.
Methods for Determining Coordination Number
The method for determining coordination number varies depending on the nature of the compound. Let's examine several approaches:
1. Simple Molecular Geometries: Visual Inspection
For simple molecules and ions with clearly defined structures, visual inspection can be the most straightforward method. This is particularly applicable to compounds with discreet molecules or ions, where the central atom's bonding partners are easily identified.
Example: In methane (CH₄), the carbon atom is at the center, and four hydrogen atoms are bonded directly to it. Therefore, the coordination number of carbon in methane is 4. Similarly, in ammonia (NH₃), nitrogen's coordination number is 3 due to its three bonds with hydrogen.
Limitations: This approach becomes impractical for complex structures with multiple ligands, extensive bonding networks, or those involving multiple central atoms.
2. Crystal Structures and X-ray Diffraction: Unveiling Solid-State Arrangements
X-ray diffraction is a powerful technique used to determine the atomic arrangement in crystalline solids. By analyzing the diffraction pattern, researchers can obtain a three-dimensional model of the crystal lattice, revealing the precise positions of atoms and their connections. From this data, the coordination number can be directly determined by counting the number of nearest neighbors surrounding a specific atom.
Example: In a rock salt (NaCl) crystal structure, each Na⁺ ion is surrounded by six Cl⁻ ions, and vice versa. Thus, both sodium and chloride ions have a coordination number of 6.
Advantages: This technique provides highly accurate and precise data, allowing the determination of coordination numbers in solids even with complex structures.
Limitations: Requires specialized equipment and expertise. The method is less suitable for amorphous or liquid materials that lack long-range order.
3. Coordination Complexes and Ligands: A Detailed Examination
Coordination complexes, involving a central metal ion surrounded by ligands, require a careful analysis of the bonding interactions. The number of ligands directly bound to the metal ion determines its coordination number. Remember that chelate ligands (ligands that bind to the metal ion at multiple points) contribute multiple bonds and hence increase the coordination number accordingly.
Example: In the complex ion [Co(NH₃)₆]³⁺, the cobalt(III) ion is surrounded by six ammonia molecules, each donating a lone pair of electrons to form a coordinate bond. Thus, the coordination number of cobalt is 6.
Example with Chelating Ligands: Consider the complex [Co(en)₃]³⁺ where 'en' represents ethylenediamine, a bidentate ligand (meaning it forms two bonds to the metal ion). Each ethylenediamine molecule contributes two bonds to the cobalt(III) ion. Therefore, the coordination number of cobalt is 3 ligands x 2 bonds/ligand = 6.
Challenges: The coordination number in coordination complexes can be influenced by factors like steric hindrance (bulky ligands preventing close approach) and electronic factors (ligand field stabilization). Determining the coordination number might necessitate advanced spectroscopic techniques.
4. Spectroscopic Techniques: Unveiling the Coordination Sphere
Several spectroscopic techniques offer insights into the coordination environment. These methods don't directly count neighbors, but provide information that allows us to infer the coordination number.
-
NMR Spectroscopy: Provides information about the local environment of atoms. The number and chemical shifts of signals can indicate the number of different types of ligands and their connectivity to the central atom.
-
IR Spectroscopy: Can reveal the presence of specific functional groups and their interactions with the central atom. Changes in vibrational frequencies can suggest the nature of the coordination bonds.
-
UV-Vis Spectroscopy: The electronic transitions in coordination complexes are highly dependent on the coordination geometry and the nature of the ligands. The absorption spectra can help determine the coordination number by comparing with known complexes with similar geometries.
-
X-ray Absorption Spectroscopy (XAS): Provides information about the local atomic structure around a specific atom. EXAFS (Extended X-ray Absorption Fine Structure) analysis can determine the number and types of neighboring atoms at different distances.
Advantages: Spectroscopic methods provide valuable complementary information about the coordination environment, confirming or refining coordination numbers derived from other techniques.
Limitations: The interpretation of spectral data can be complex and requires considerable expertise.
5. Considering Oxidation State and Electronic Configuration: Predictive Approach
In some cases, understanding the oxidation state and electronic configuration of the central atom can offer a predictive estimate of the coordination number. This is often used in conjunction with other data, like ligand properties. Transition metals, for example, tend to exhibit varying coordination numbers depending on the electronic configuration and the nature of the ligands. However, this method should be considered a preliminary guess rather than a definitive determination.
Example: Consider transition metal ions with d8 electronic configuration, like palladium (II). They often exhibit square planar geometry (CN=4) or tetrahedral geometry (CN=4), though other geometries are also possible.
Limitations: This approach provides only a general prediction and requires additional evidence to confirm the coordination number.
Advanced Considerations and Complex Scenarios
Determining the coordination number in complex situations might require a combination of the techniques discussed above.
-
Polymeric Structures: In polymeric materials, determining the coordination number involves identifying the number of atoms directly bonded to the central atom within the repeating unit of the polymer. The coordination number can vary depending on the position of the atom in the polymer chain.
-
Fluxionality and Dynamic Systems: In some cases, the coordination number might not be static. Ligands might exchange rapidly, leading to an average coordination number. Advanced dynamic NMR techniques are needed to resolve these situations.
-
Mixed Ligand Complexes: Compounds containing multiple types of ligands require a more careful analysis of the binding modes of each ligand to determine the total coordination number.
-
Ambiguous Bonding: In certain systems, distinguishing between direct bonding and weaker interactions (e.g., hydrogen bonds) might be challenging. Careful consideration of bond lengths and other physical properties is crucial in these cases.
Conclusion: A Multifaceted Approach
Determining the coordination number of a central atom requires a multifaceted approach, combining visual inspection with powerful analytical techniques. While simple cases can be addressed through direct observation, complex systems necessitate a comprehensive analysis using X-ray diffraction, various spectroscopic methods, and a deep understanding of bonding principles and chemical structure. Combining several approaches provides the most reliable and complete picture of the coordination environment. Remember that the coordination number is a fundamental property that plays a vital role in understanding the structural, electronic, and reactivity characteristics of a compound. A thorough understanding of its determination is therefore essential for anyone involved in inorganic or materials chemistry.
Latest Posts
Latest Posts
-
Is Nitrogen Metal Nonmetal Or Metalloid
Mar 09, 2025
-
How To Find Square Root Of Fraction
Mar 09, 2025
-
What Are The Factors Of 53
Mar 09, 2025
-
What Is A Factor Of 38
Mar 09, 2025
-
What Are The Common Factors Of 14 And 35
Mar 09, 2025
Related Post
Thank you for visiting our website which covers about How To Find A Coordination Number . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
