Which Of The Following Determines The Identity Of An Element
Juapaving
Apr 07, 2025 · 5 min read
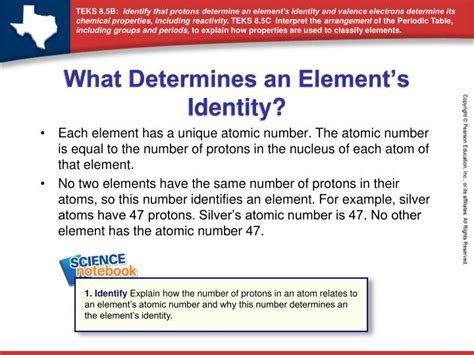
Table of Contents
Which of the Following Determines the Identity of an Element?
The identity of a chemical element is a fundamental concept in chemistry. Understanding what defines an element is crucial to grasping the complexities of chemical reactions, bonding, and the periodic table itself. While several properties might seem to distinguish one element from another, only one definitively determines its identity: the number of protons in its nucleus. Let's delve deeper into this, exploring why proton number reigns supreme and examining other properties that, while characteristic, are not defining factors.
The Proton: The Defining Characteristic of an Element
An element's identity is inextricably linked to its atomic structure, specifically the number of protons found in its nucleus. This number is known as the atomic number and is represented by the symbol Z. It's the atomic number, and only the atomic number, that uniquely identifies an element. Hydrogen (Z=1), with one proton, is fundamentally different from helium (Z=2), with two protons, and this difference is absolute. No matter the other properties, a change in the number of protons transforms one element into another.
Why Not Other Subatomic Particles?
While the nucleus also contains neutrons, the number of neutrons can vary within a given element, leading to isotopes. Isotopes of the same element have the same number of protons but differ in their neutron count. For instance, carbon-12 (¹²C) and carbon-14 (¹⁴C) are both carbon because they both have six protons. However, ¹²C has six neutrons, while ¹⁴C has eight. This difference in neutron number affects the isotope's stability and mass but doesn't alter its elemental identity.
Electrons, residing in orbitals surrounding the nucleus, are also crucial for an atom's behavior, particularly its chemical reactivity. The number of electrons usually equals the number of protons in a neutral atom. However, atoms can gain or lose electrons, forming ions with different charges. These ions have different properties than the neutral atom, but they remain the same element. A sodium ion (Na⁺), which has lost one electron, is still sodium; it hasn't transformed into another element.
Therefore, it's clear that neither the number of neutrons nor the number of electrons defines the identity of an element. Only the unchanging number of protons – the atomic number – dictates which element an atom belongs to.
Properties That Correlate With, But Don't Define, Element Identity
Several properties are closely related to an element's atomic number and are therefore characteristic of that element. However, these are consequences of the proton number, not the defining factor itself. Let's look at some of them:
1. Atomic Mass: A Weighted Average
Atomic mass, often confused with atomic number, is the average mass of all the isotopes of an element, weighted by their abundance in nature. This is not a unique identifier, as different elements can have similar atomic masses. For example, gold (Au) and mercury (Hg) have atomic masses close to 200 amu but are distinctly different elements. Atomic mass is a consequence of the number of protons and neutrons and their relative abundances, not the determinant of elemental identity.
2. Chemical Properties: Reactivity and Bonding Behavior
The chemical properties of an element – its ability to react with other substances and the types of bonds it forms – are directly influenced by its electron configuration, which is, in turn, determined by the number of protons. Elements in the same group (column) of the periodic table have similar chemical properties due to similar electron configurations in their outermost shells (valence electrons). However, while chemical properties are highly characteristic, they don't define the element. Two different elements could, theoretically, display similar chemical behavior under specific conditions. Therefore, reactivity is a consequence, not a defining characteristic.
3. Physical Properties: Appearance and Behavior
Physical properties like melting point, boiling point, density, color, and conductivity also vary significantly across elements. These properties depend on the strength of interatomic forces, atomic size, and electron configuration – all of which are related to the number of protons. However, several elements can share similar physical properties, making them unreliable for unique identification. The observation of a physical property does not guarantee an element's identity.
4. Spectral Lines: A Fingerprint of Energy Levels
Each element emits or absorbs light at specific wavelengths, producing unique spectral lines. This phenomenon is due to the distinct energy levels of its electrons, which are a consequence of the number of protons and the resulting electron configuration. Spectral analysis is a powerful tool for identifying elements, but the spectral lines themselves are a result of the atomic structure, not the fundamental defining factor.
The Importance of Understanding Elemental Identity
Understanding what truly defines an element—its atomic number—is crucial for numerous reasons:
-
Predicting Chemical Behavior: Knowing the atomic number allows chemists to predict an element's reactivity and how it will interact with other elements. This is the basis of chemical bonding and reaction prediction.
-
Organizing the Periodic Table: The periodic table is organized based on increasing atomic number, reflecting the periodic trends in chemical and physical properties. This organization facilitates understanding the relationships between different elements.
-
Nuclear Chemistry and Isotopes: In nuclear chemistry, understanding the role of protons and neutrons is vital for studying radioactive decay, nuclear fission, and fusion processes. The concept of isotopes and their behavior is directly tied to the number of protons and neutrons.
-
Material Science and Engineering: The properties of materials are largely determined by the elements they contain and their atomic structure. The atomic number is fundamental to designing materials with specific properties.
-
Analytical Chemistry: Various analytical techniques, like spectroscopy and chromatography, rely on the unique characteristics of elements related to their atomic numbers for identification and quantification.
Conclusion: The Unchanging Proton
In summary, while many properties are characteristic of a specific element and help in its identification, only the number of protons in the nucleus – the atomic number – definitively determines the identity of a chemical element. Other properties, such as atomic mass, chemical behavior, physical properties, and spectral lines, are consequences of the proton number and the resultant atomic structure. Therefore, the atomic number is the single, unvarying, and fundamental property that sets one element apart from all others. Mastering this concept is crucial for a thorough understanding of chemistry and its vast applications in various scientific fields.
Latest Posts
Latest Posts
-
2 3 4 As An Improper Fraction
Apr 08, 2025
-
Find The Gcf Of 8 And 12
Apr 08, 2025
-
What Is 0 5 As A Percentage
Apr 08, 2025
-
What Are The 3 Main Types Of Electoral Systems
Apr 08, 2025
-
What Is The Difference Between Glycogen And Starch
Apr 08, 2025
Related Post
Thank you for visiting our website which covers about Which Of The Following Determines The Identity Of An Element . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.