What Is The Difference Between Glycogen And Starch
Juapaving
Apr 08, 2025 · 6 min read
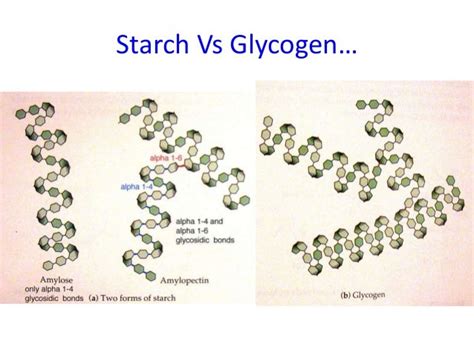
Table of Contents
What's the Difference Between Glycogen and Starch? A Deep Dive into Energy Storage
Both glycogen and starch serve as crucial energy storage molecules in living organisms, but their structural differences dictate their distinct roles and properties. Understanding these differences is fundamental to comprehending biological energy metabolism and various applications in food science and biotechnology. This article delves deep into the molecular structures, functions, and key distinctions between glycogen and starch.
Structural Variations: The Molecular Blueprint
Glycogen and starch are both polysaccharides, meaning they are large polymers composed of numerous monosaccharide units linked together. However, the type of monosaccharide and the nature of the glycosidic bonds significantly differentiate them.
Glycogen: The Animal Energy Store
Glycogen, primarily found in animals and fungi, is a highly branched polymer of glucose. This means its building blocks are glucose molecules. These glucose units are linked by two types of glycosidic bonds:
- α-1,4-glycosidic bonds: These bonds connect glucose molecules in a linear chain.
- α-1,6-glycosidic bonds: These bonds create branch points, forming the highly branched structure characteristic of glycogen.
The branching in glycogen is extensive, with branches occurring roughly every 8-12 glucose units. This highly branched structure is crucial for its function. The many non-reducing ends (the ends where glucose can be added or removed) allow for rapid glucose mobilization when energy is needed. Imagine it like a tree with many branches—it's easier to harvest leaves (glucose) from many branches simultaneously than from a single, long trunk.
Starch: The Plant's Carbohydrate Reservoir
Starch, the primary energy storage molecule in plants, is also a glucose polymer but with a less branched structure than glycogen. It exists in two main forms:
- Amylose: This is a linear polymer of glucose units connected by α-1,4-glycosidic bonds. It forms a helical structure. Think of it like a long, unbranched chain.
- Amylopectin: Similar to glycogen, amylopectin is a branched polymer of glucose. However, its branching is less frequent than in glycogen, occurring approximately every 24-30 glucose units. The branch points are also formed by α-1,6-glycosidic bonds.
Functional Differences: Mobilizing Energy
The structural differences between glycogen and starch directly impact their functionality in energy metabolism.
Glycogen's Rapid Energy Release
Glycogen's highly branched structure is perfectly adapted for rapid glucose release. When energy is required, enzymes can simultaneously access and break down glucose molecules from numerous non-reducing ends. This allows for a quick surge of glucose, providing immediate energy for cellular processes. This is especially important in animals that require rapid bursts of energy, such as during "fight-or-flight" responses. The rapid mobilization ensures that energy is readily available when needed.
Starch's Gradual Energy Supply
Starch, with its less branched structure (especially amylose), provides a more gradual release of glucose. Amylose's linear structure makes it less accessible to enzymes compared to glycogen's highly branched structure. The breakdown of starch is slower, providing a sustained release of glucose for plant metabolic needs. This slow-release mechanism is advantageous for plants that need a constant supply of energy throughout their growth cycle.
Location and Synthesis: Where and How They're Made
The synthesis and storage locations of glycogen and starch also differ, reflecting their roles in different organisms.
Glycogen Synthesis and Storage
Glycogen is primarily synthesized and stored in the liver and muscles of animals. The liver acts as a central glucose reservoir, regulating blood glucose levels by releasing glucose into the bloodstream when needed. Muscle glycogen serves as a local energy source for muscle contraction. The synthesis involves a complex pathway requiring several enzymes, starting with glucose-6-phosphate, which is then converted into glucose-1-phosphate, and ultimately into glycogen.
Starch Synthesis and Storage
Starch is synthesized in chloroplasts of plant cells, particularly in leaves and storage organs such as seeds, tubers, and roots. The synthesis begins with glucose produced during photosynthesis and involves the concerted action of several enzymes that catalyze the formation of both amylose and amylopectin. These are then stored within starch granules within the plant cells.
Chemical Properties: Solubility and Digestion
The chemical properties of glycogen and starch also differ, influencing their solubility and digestibility.
Solubility
Glycogen is highly soluble in water, forming a colloid solution. This property enables its efficient dissolution and distribution in the cellular environment. Starch, on the other hand, is relatively insoluble in cold water but partially soluble in hot water, forming a paste or gel. This difference in solubility is related to the degree of branching and the interactions between the polymer chains.
Digestion
Both glycogen and starch are digested by enzymes called amylases. Amylases break down the α-1,4-glycosidic bonds, releasing glucose units. However, the digestibility differs due to the structural variations. Glycogen's highly branched structure makes it more readily digestible compared to starch, particularly amylose. The greater number of non-reducing ends allows for more simultaneous enzymatic action and rapid glucose release. The branching in amylopectin also facilitates faster digestion compared to the linear amylose.
Applications: Beyond Biological Roles
The unique properties of glycogen and starch also lead to various applications in different fields.
Glycogen in Food and Pharmaceuticals
Although not as extensively used as starch, glycogen finds applications in food products as a thickener or stabilizer. Its high water solubility makes it useful in certain food formulations. It’s also being explored in pharmaceutical contexts for its potential roles in drug delivery systems and as a biocompatible material.
Starch's Wide-Ranging Applications
Starch boasts a wide array of industrial applications. Its properties make it a versatile ingredient in various products, including:
- Food industry: Starch is a crucial ingredient in many food products, serving as a thickener, binder, stabilizer, and texturizer in sauces, soups, baked goods, and processed foods.
- Textile industry: Starch is used as a sizing agent in textiles, coating yarns to increase their strength and prevent breakage during weaving.
- Paper industry: Starch is used as a binder and coating agent in paper manufacturing, improving its strength and surface properties.
- Bioplastics: Starch is increasingly used as a raw material for producing biodegradable plastics, offering a sustainable alternative to traditional plastics derived from petroleum.
Summary Table: Key Differences at a Glance
| Feature | Glycogen | Starch |
|---|---|---|
| Source | Animals, fungi | Plants |
| Structure | Highly branched | Linear (amylose) and branched (amylopectin) |
| Branching | Frequent (every 8-12 glucose units) | Less frequent (amylopectin: every 24-30) |
| Glucose Release | Rapid | Gradual |
| Solubility | Highly soluble in water | Partially soluble (hot water) |
| Digestibility | Highly digestible | Less digestible (amylose) |
| Storage Location | Liver, muscles | Chloroplasts, storage organs |
Conclusion: Understanding the Nuances
The differences between glycogen and starch extend beyond their simple chemical compositions. Their structural variations have profound implications for their functions as energy storage molecules, their solubility, digestibility, and their respective applications in diverse fields. A thorough understanding of these differences is crucial for researchers in biology, food science, and biotechnology, paving the way for innovation in various areas, from developing improved food products to creating sustainable biomaterials. Further research into the detailed mechanisms of synthesis, degradation, and applications of both glycogen and starch will undoubtedly continue to expand our knowledge and offer exciting possibilities in the future.
Latest Posts
Latest Posts
-
Whats The Lcm Of 9 And 12
Apr 08, 2025
-
Which Is Not One Of The Five Pillars Of Islam
Apr 08, 2025
-
How Many Feet Is 65 In
Apr 08, 2025
-
5 Letter Words Beginning With Ae
Apr 08, 2025
-
The Most Abundant Metal In The Earths Crust Is
Apr 08, 2025
Related Post
Thank you for visiting our website which covers about What Is The Difference Between Glycogen And Starch . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.