What Unit Do You Use To Measure Thermal Energy
Juapaving
Mar 30, 2025 · 6 min read
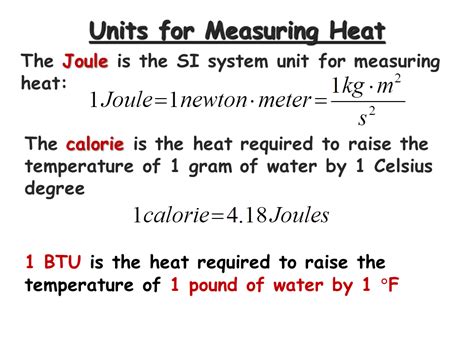
Table of Contents
What Unit Do You Use to Measure Thermal Energy? A Comprehensive Guide
Thermal energy, also known as heat, is a fundamental concept in physics and engineering. Understanding how to measure it accurately is crucial in numerous applications, from designing efficient heating systems to analyzing the performance of power plants. But what unit do you use to measure thermal energy? The answer isn't as simple as a single unit, as the choice depends on the context and the specific application. This comprehensive guide will delve into the various units used to quantify thermal energy, explaining their relationships and when each is most appropriate.
The Joule: The Foundation of Thermal Energy Measurement
The Joule (J) is the fundamental SI unit of energy, including thermal energy. One joule is defined as the work done when a force of one newton is applied over a distance of one meter. While seemingly unrelated to heat, the Joule’s significance lies in the first law of thermodynamics, which states that energy cannot be created or destroyed, only transferred or transformed. This principle directly links mechanical work and heat, establishing the Joule as the fundamental unit for both. This means any form of energy, including thermal energy, can be expressed in Joules.
Why Joules are Universal
The universality of the Joule makes it ideal for scientific calculations and theoretical understanding. Whether you're dealing with the heat generated by friction, the energy released during a chemical reaction, or the thermal energy transferred in a heat exchanger, the Joule provides a consistent and standardized measure. This consistency is vital for ensuring accurate comparisons and calculations across different systems and experiments. Furthermore, the Joule's relationship to other SI units (Newtons, meters, seconds) allows for seamless integration into broader physics equations.
The Calorie and Kilocalorie: Historical and Practical Units
The calorie (cal) and kilocalorie (kcal), also known as the large calorie or Calorie (with a capital "C"), are historically significant units of thermal energy. One calorie was originally defined as the amount of heat required to raise the temperature of one gram of water by one degree Celsius at standard atmospheric pressure. This definition highlights the connection between heat and temperature change, a key aspect of many thermal energy applications.
The kilocalorie (kcal or Calorie), equal to 1000 calories, is often used to measure the energy content of food. This is due to the historical development of calorimetry, which involved measuring the heat released by burning food to determine its energy content. While still used in some contexts, the calorie and kilocalorie are gradually being replaced by the Joule in scientific work due to the SI system's widespread adoption.
The Relationship Between Joules and Calories
The conversion factor between Joules and calories is approximately:
- 1 cal ≈ 4.184 J
- 1 kcal ≈ 4184 J
Understanding this conversion is crucial when working with older literature or data that utilizes calories. Always ensure consistent units throughout your calculations to avoid errors.
British Thermal Unit (BTU): A Common Unit in Engineering
The British Thermal Unit (BTU) is another unit of thermal energy frequently used in engineering and HVAC (Heating, Ventilation, and Air Conditioning) applications. One BTU is defined as the amount of heat required to raise the temperature of one pound of water by one degree Fahrenheit. Similar to the calorie, the BTU's definition directly relates to a temperature change in a specific substance.
The BTU's prevalence in engineering stems from its historical use in the United States and other countries that employ the Imperial system of units. While the Joule is the preferred unit in scientific contexts, the BTU remains a practical unit for many engineering calculations involving heating and cooling systems.
BTU to Joule Conversion
The conversion factor between BTUs and Joules is approximately:
- 1 BTU ≈ 1055 J
Similar to calories, always ensure consistent units throughout your calculations when using BTUs to avoid inaccuracies.
Choosing the Right Unit for Your Application
The choice of unit for measuring thermal energy depends heavily on the context and application.
-
Scientific Research and Theoretical Physics: The Joule is the universally preferred unit due to its fundamental nature and integration within the SI system.
-
Food Science and Nutrition: The kilocalorie (Calorie) remains commonly used for labeling the energy content of food.
-
HVAC and Engineering Applications: The BTU is often used for calculations involving heating and cooling systems, especially in regions where the Imperial system is prevalent.
-
Everyday Applications: While not a formal unit, the expression "units of heat" is sometimes used informally to refer to energy, but this lacks the precision of the aforementioned units.
Regardless of the unit chosen, it's crucial to maintain consistency throughout any calculation or analysis to ensure accuracy and prevent errors. Conversion factors should be applied appropriately when dealing with multiple units in the same calculation.
Advanced Concepts and Applications
The measurement of thermal energy extends beyond the basic units discussed above. Understanding heat transfer mechanisms, such as conduction, convection, and radiation, necessitates more nuanced approaches.
Specific Heat Capacity
Specific heat capacity is a crucial concept related to thermal energy. It represents the amount of heat required to raise the temperature of one unit mass of a substance by one degree. The unit of specific heat capacity often involves Joules, calories, or BTUs, coupled with units of mass (kilograms, grams, or pounds) and temperature (Celsius or Kelvin, Fahrenheit). This parameter is essential for predicting temperature changes in various materials in response to heat transfer.
Latent Heat
Latent heat refers to the energy absorbed or released during phase transitions (e.g., melting, boiling, freezing). This energy change doesn't result in a temperature increase, unlike the heat described in specific heat capacity. Latent heat is also measured in Joules, calories, or BTUs, reflecting the total energy involved in the phase transformation. Understanding latent heat is vital in applications involving refrigeration, steam generation, and material processing.
Thermodynamic Processes
The measurement of thermal energy plays a central role in thermodynamics. Analyzing thermodynamic processes like adiabatic expansion, isothermal compression, or isobaric heating involves careful tracking of heat transfer, work done, and internal energy changes. All these quantities are ultimately expressed using units of energy, primarily the Joule.
Heat Flux and Heat Transfer Rate
Heat flux, the rate of heat transfer per unit area, is often expressed in Watts per square meter (W/m²). This unit connects thermal energy transfer with time and area, providing crucial information for applications like thermal insulation design, heat exchanger analysis, and electronic cooling. The heat transfer rate, which measures the energy transferred per unit time, is measured in Watts (W), equivalent to Joules per second (J/s).
Conclusion
Measuring thermal energy is a crucial aspect of numerous scientific, engineering, and everyday applications. While the Joule stands as the fundamental SI unit, the calorie, kilocalorie, and BTU remain relevant in specific contexts. Choosing the appropriate unit requires understanding the application and maintaining consistency throughout calculations. Furthermore, the concepts of specific heat capacity, latent heat, and heat transfer rate enrich our understanding of thermal energy and its implications in diverse fields. As scientific understanding evolves, the Joule's prominence as the fundamental unit of energy will likely continue to grow, further solidifying its importance in the precise and consistent measurement of thermal energy.
Latest Posts
Latest Posts
-
How Many Rna Polymerases Are Found In Prokaryotes
Apr 01, 2025
-
When Two Parallel Lines Are Crossed By A Transversal
Apr 01, 2025
-
Device That Converts Light Energy Into Electrical Energy
Apr 01, 2025
-
Relationship Between Degree Celsius And Fahrenheit
Apr 01, 2025
-
What Is The Lcm Of 26 And 39
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about What Unit Do You Use To Measure Thermal Energy . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
