What Is The Si Unit Of Measurement For Temperature
Juapaving
Apr 07, 2025 · 6 min read
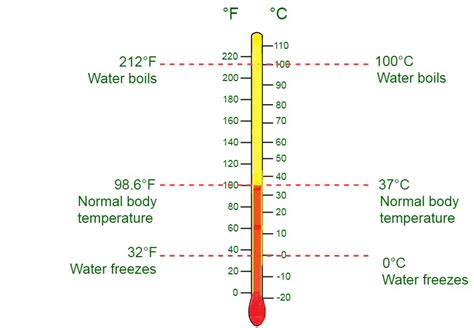
Table of Contents
What is the SI Unit of Measurement for Temperature?
The world of measurement is vast and intricate, governing everything from the minuscule size of an atom to the colossal distances between galaxies. Within this realm, temperature stands as a fundamental physical quantity, reflecting the average kinetic energy of the particles within a system. Understanding temperature measurement is crucial across numerous fields, from cooking and meteorology to advanced physics and engineering. This comprehensive guide delves into the SI unit of temperature, exploring its history, applications, and significance in scientific understanding.
The Kelvin: The SI Unit of Thermodynamic Temperature
The International System of Units (SI), the modern form of the metric system, defines seven base units for fundamental physical quantities. For temperature, the SI unit is the kelvin (K), named after the eminent physicist Lord Kelvin (William Thomson). It's crucial to understand that the kelvin measures thermodynamic temperature, a fundamental property of matter reflecting the total internal energy of a system. This is different from other temperature scales, which are relative scales.
Understanding Thermodynamic Temperature
Thermodynamic temperature is a scale that is absolute; it starts at absolute zero, the theoretical point where all molecular motion ceases. This contrasts with scales like Celsius and Fahrenheit, which are relative, meaning their zero points are arbitrarily defined. Absolute zero is approximately -273.15°C or -459.67°F. The importance of thermodynamic temperature lies in its direct correlation with the internal energy of a system. A higher Kelvin temperature directly translates to higher average kinetic energy of the particles comprising the system.
The Definition of the Kelvin
The kelvin is defined based on the Boltzmann constant (k<sub>B</sub>), a fundamental physical constant relating the average kinetic energy of particles in a gas to its absolute temperature. The current definition links the kelvin to the Boltzmann constant and the triple point of water. The triple point is the unique temperature and pressure at which water coexists in its three phases: solid (ice), liquid (water), and gas (water vapor). This definition ensures high accuracy and reproducibility of the kelvin.
Relationship between Kelvin, Celsius, and Fahrenheit
While the kelvin is the SI unit, other temperature scales are commonly used, particularly Celsius (°C) and Fahrenheit (°F). Understanding their relationships with the kelvin is essential for accurate conversions and scientific calculations.
Kelvin and Celsius: A Simple Conversion
The Celsius scale is defined such that 0°C corresponds to the freezing point of water and 100°C to its boiling point at standard atmospheric pressure. The relationship between Kelvin and Celsius is straightforward:
K = °C + 273.15
This means that to convert a Celsius temperature to Kelvin, simply add 273.15. Conversely, to convert from Kelvin to Celsius, subtract 273.15.
Kelvin and Fahrenheit: A More Complex Conversion
The Fahrenheit scale, primarily used in the United States, has a different zero point and degree size compared to Celsius and Kelvin. The conversion formulas are slightly more complex:
K = (°F + 459.67) × 5/9
°F = (K × 9/5) - 459.67
These formulas require multiplying and dividing by constants to account for the different scales.
Applications of the Kelvin Scale
The Kelvin scale's significance extends far beyond simple temperature measurements. Its absolute nature makes it indispensable across various scientific and engineering disciplines:
1. Thermodynamics and Statistical Mechanics:
The Kelvin scale is foundational to thermodynamics and statistical mechanics. The laws of thermodynamics are inherently expressed in terms of absolute temperature, allowing precise analysis of energy transfer and entropy changes.
2. Gas Laws:
Many gas laws, such as the ideal gas law (PV = nRT), require temperature to be expressed in Kelvin. The absolute temperature scale ensures accurate predictions of gas behavior under varying conditions.
3. Astronomy and Astrophysics:
In astronomy and astrophysics, the Kelvin scale is used to measure the temperatures of stars, planets, and interstellar gas clouds. The extremely high temperatures involved necessitate the use of an absolute scale for meaningful measurements and theoretical analysis.
4. Material Science and Engineering:
Material properties, such as thermal expansion and conductivity, are temperature-dependent. Precise Kelvin measurements are critical for designing and characterizing materials with desired properties, particularly at extreme temperatures.
5. Cryogenics and Superconductivity:
Cryogenics, the study of extremely low temperatures, relies heavily on the Kelvin scale. Many materials exhibit unique properties, such as superconductivity, at temperatures close to absolute zero, making precise Kelvin measurement crucial.
6. Climate Science and Meteorology:
While Celsius might be reported to the public, Kelvin is frequently used in climate modeling and research to understand atmospheric processes, temperature trends, and climate change. The absolute nature of the Kelvin scale is vital for accurate calculations and simulations.
Importance of Accurate Temperature Measurement
The accuracy of temperature measurement significantly impacts numerous applications. Inaccurate measurements can lead to:
- Experimental errors: Inaccurate temperature readings in scientific experiments can lead to flawed results and conclusions.
- Engineering failures: Incorrect temperature calculations in engineering design can result in structural failures and safety hazards.
- Medical misdiagnosis: Inaccurate body temperature readings can lead to misdiagnosis and ineffective treatment in healthcare.
- Industrial inefficiencies: Inaccurate temperature control in industrial processes can lead to reduced efficiency, product defects, and increased energy consumption.
Consequently, precise and reliable temperature measurement devices and techniques are vital across various sectors.
Advances in Thermometry
Modern thermometry has seen significant advancements, improving the accuracy and precision of temperature measurement. This includes:
- Development of advanced sensors: Thermocouples, resistance temperature detectors (RTDs), and thermistors provide highly accurate and precise temperature readings over a wide range.
- Improved calibration techniques: Sophisticated calibration methods ensure that temperature measurement instruments provide reliable and traceable results.
- Digital thermometry: Digital thermometers provide rapid and accurate temperature readings with minimal human error.
- Remote sensing: Remote temperature sensing technologies enable measurement in hazardous or inaccessible environments.
Conclusion: The Kelvin's Enduring Significance
The kelvin, the SI unit of thermodynamic temperature, plays a pivotal role in our understanding of the physical world. Its absolute nature and precise definition make it indispensable across a vast array of scientific and engineering fields. From understanding the behavior of gases to exploring the cosmos, the kelvin provides a fundamental framework for accurate temperature measurement and analysis. As technology continues to evolve, the accuracy and precision of Kelvin-based measurements will remain crucial for scientific advancement and technological progress. The relentless pursuit of ever-more-precise temperature measurements underscores the continuing importance of the kelvin as a cornerstone of the international system of units. The quest for a deeper understanding of the universe and the materials that comprise it hinges, in part, on the ability to accurately and reliably measure temperature using the fundamental unit, the Kelvin.
Latest Posts
Latest Posts
-
Lcm Of 3 6 And 7
Apr 08, 2025
-
The Three Medians Of A Triangle Intersect At The
Apr 08, 2025
-
What Is The Difference Between Cell Wall And Cell Membrane
Apr 08, 2025
-
How Many Hours In A Leap Year
Apr 08, 2025
-
What Is The Lcm Of 6 12 And 15
Apr 08, 2025
Related Post
Thank you for visiting our website which covers about What Is The Si Unit Of Measurement For Temperature . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.