What Is The Hybridization Of The Central Atom In Sf4
Juapaving
Mar 30, 2025 · 6 min read
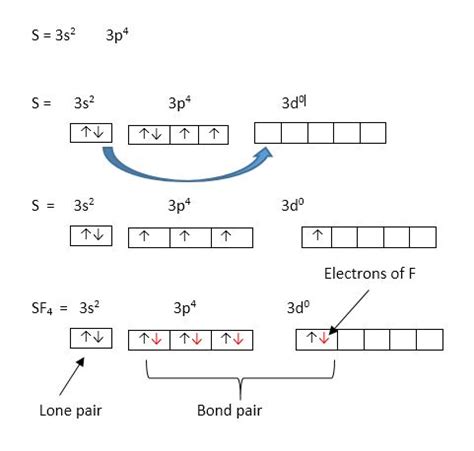
Table of Contents
What is the Hybridization of the Central Atom in SF₄? A Deep Dive into Sulfur Tetrafluoride's Molecular Geometry
Understanding the hybridization of the central atom in a molecule is crucial for predicting its geometry and properties. Sulfur tetrafluoride (SF₄), a fascinating inorganic compound, presents a compelling case study in this area. This article will delve deeply into the hybridization of sulfur in SF₄, explaining the process, the resulting molecular geometry, and the implications of this hybridization for the molecule's reactivity and overall behavior.
Understanding Hybridization: A Quick Recap
Before we dive into the specifics of SF₄, let's briefly review the concept of hybridization. Hybridization is a model used in chemistry to explain the bonding in molecules that cannot be adequately explained by simple valence bond theory. It posits that atomic orbitals of similar energy combine to form hybrid orbitals, which then participate in bonding. These hybrid orbitals are different from the original atomic orbitals in terms of shape and energy. The most common types of hybridization involve s and p orbitals, leading to sp, sp², sp³, and sp³d hybrid orbitals. The type of hybridization depends on the number of sigma bonds and lone pairs around the central atom.
Determining the Hybridization of Sulfur in SF₄
To determine the hybridization of sulfur in SF₄, we follow a systematic approach:
1. Draw the Lewis Structure:
The Lewis structure of SF₄ shows sulfur (S) as the central atom, bonded to four fluorine (F) atoms. Sulfur has six valence electrons, and each fluorine atom contributes one electron for bonding. This leaves one lone pair of electrons on the sulfur atom.
F
|
F-S-F
|
F
|
Lone Pair
2. Count the Steric Number:
The steric number is the sum of the number of sigma bonds and lone pairs around the central atom. In SF₄, sulfur forms four sigma bonds with four fluorine atoms and has one lone pair. Therefore, the steric number is 5 (4 sigma bonds + 1 lone pair).
3. Determine the Hybridization:
The steric number directly relates to the type of hybridization. A steric number of 5 corresponds to sp³d hybridization. This means that one s orbital, three p orbitals, and one d orbital from the sulfur atom combine to form five sp³d hybrid orbitals. These hybrid orbitals are then used to form four sigma bonds with the fluorine atoms and to accommodate the lone pair.
The Molecular Geometry of SF₄: A Consequence of sp³d Hybridization
The sp³d hybridization in SF₄ leads to a specific molecular geometry. While the electron-pair geometry (considering both bonding pairs and lone pairs) is trigonal bipyramidal, the molecular geometry (considering only the positions of the atoms) is see-saw or disphenoidal. This is because the lone pair occupies an equatorial position, which exerts a greater repulsive force on the bonding pairs compared to an axial position. This repulsion pushes the axial fluorine atoms closer together, resulting in the characteristic see-saw shape.
Visualizing the See-Saw Geometry
Imagine a see-saw. The sulfur atom sits at the fulcrum, two fluorine atoms are at the ends of the see-saw (axial positions), and the other two fluorine atoms are at the points where a child would sit (equatorial positions). The lone pair occupies the remaining equatorial position.
The Role of d Orbitals in sp³d Hybridization
The involvement of a d orbital in the sp³d hybridization of sulfur in SF₄ is noteworthy. Sulfur, being a third-row element, possesses readily available d orbitals that can participate in hybridization. This contrasts with second-row elements like oxygen and nitrogen, which lack easily accessible d orbitals and typically do not undergo hybridization involving d orbitals. The participation of a d-orbital allows sulfur to expand its octet, accommodating more than eight electrons in its valence shell.
Implications of sp³d Hybridization in SF₄
The sp³d hybridization of sulfur in SF₄ has several important implications for its properties:
-
Polarity: The see-saw geometry and the presence of a lone pair make SF₄ a polar molecule. The dipole moments of the S-F bonds do not cancel each other out due to the asymmetrical arrangement.
-
Reactivity: The presence of a lone pair on the sulfur atom makes SF₄ a Lewis base, capable of donating its lone pair to electron-deficient species. This contributes to its reactivity, allowing it to participate in various reactions such as Lewis acid-base reactions.
-
Bond Angles: The bond angles in SF₄ deviate from the ideal angles predicted for a perfectly symmetrical trigonal bipyramidal structure. The axial F-S-F bond angle is less than 180°, and the equatorial F-S-F bond angles are less than 120° due to the lone pair repulsion.
-
Stability: Despite the presence of a highly electronegative fluorine atom, SF₄ is a relatively stable compound, at least at moderate temperatures. The strong S-F bonds and the overall molecular structure contribute to its stability.
Comparing SF₄ Hybridization to other Sulfur Fluorides
It's instructive to compare the hybridization of sulfur in SF₄ to other sulfur fluorides:
-
SF₆: Sulfur hexafluoride (SF₆) has a steric number of 6 (six sigma bonds and no lone pairs), resulting in sp³d² hybridization and an octahedral geometry. It is a nonpolar molecule.
-
SF₂: Sulfur difluoride (SF₂) has a steric number of 4 (two sigma bonds and two lone pairs), resulting in sp³ hybridization and a bent geometry. It's a polar molecule.
This comparison highlights how the number of bonding pairs and lone pairs significantly influences the hybridization and, consequently, the geometry and properties of the molecule.
Advanced Considerations: Beyond Simple Hybridization Models
While the sp³d hybridization model provides a useful framework for understanding the bonding in SF₄, it's important to acknowledge its limitations. More sophisticated computational methods, such as density functional theory (DFT) calculations, offer a more nuanced picture of the electronic structure and bonding in this molecule. These methods may reveal contributions from other orbitals and a more complex bonding picture than the simplified sp³d model.
Conclusion: A Powerful Tool for Prediction
The hybridization of the central atom in a molecule is a powerful concept for predicting its molecular geometry and understanding its properties. In the case of SF₄, the sp³d hybridization of sulfur leads to a see-saw molecular geometry, a polar molecule with unique reactivity characteristics, and relative stability. Understanding this hybridization model is crucial for predicting and interpreting the behavior of this and other similar molecules. While simplified models like sp³d hybridization are excellent starting points, remembering the limitations and seeking more sophisticated methods for a deeper understanding can provide a richer and more complete picture of molecular structure and bonding. The exploration of SF₄'s hybridization serves as a valuable illustration of the power and limitations of these models in the field of inorganic chemistry.
Latest Posts
Latest Posts
-
Least Common Multiple 3 And 8
Apr 01, 2025
-
Is Tearing Paper A Physical Change
Apr 01, 2025
-
The Movement Of Water Across A Semipermeable Membrane Is Called
Apr 01, 2025
-
Differences Between Primary Data And Secondary Data
Apr 01, 2025
-
Where Glucose Gets Broken Into Pyruvate In The Cell
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about What Is The Hybridization Of The Central Atom In Sf4 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
