The Movement Of Water Across A Semipermeable Membrane Is Called
Juapaving
Apr 01, 2025 · 7 min read
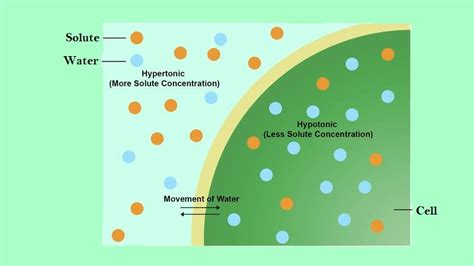
Table of Contents
The Movement of Water Across a Semipermeable Membrane is Called Osmosis: A Deep Dive
The movement of water across a semipermeable membrane is called osmosis. This seemingly simple process is fundamental to life itself, driving countless biological functions from the transport of nutrients in plants to the regulation of blood pressure in animals. Understanding osmosis requires delving into the intricacies of semipermeable membranes, concentration gradients, and the driving force behind water's movement. This comprehensive guide will explore these concepts, providing a detailed explanation of osmosis, its types, and its significance in various biological systems.
Understanding Semipermeable Membranes
Before diving into the specifics of osmosis, it's crucial to understand the role of the semipermeable membrane. This specialized membrane acts as a selective barrier, allowing some substances to pass through while restricting others. This selectivity is based on factors such as size, charge, and solubility of the molecules. In biological systems, the cell membrane is the primary example of a semipermeable membrane. It's composed of a phospholipid bilayer, interspersed with proteins and other molecules that regulate the passage of substances. This membrane allows the free passage of water molecules while restricting the movement of larger molecules like proteins and sugars. This selective permeability is what makes osmosis possible.
The Structure of Semipermeable Membranes: A Closer Look
The specific composition of a semipermeable membrane dictates its permeability characteristics. In biological systems, the cell membrane's lipid bilayer forms a hydrophobic barrier to many hydrophilic molecules. However, specialized protein channels and pores embedded within this bilayer allow controlled transport of specific molecules and ions. These channels and pores can be gated, meaning their opening and closing are regulated based on cellular needs. Artificial semipermeable membranes, often used in experiments, can be made from materials such as cellulose acetate, which possess different pore sizes and thus different permeability properties.
Osmosis: The Driving Force
Osmosis is the passive movement of water across a selectively permeable membrane from a region of high water concentration to a region of low water concentration. This movement continues until an equilibrium is reached, where the water concentration is equal on both sides of the membrane. Crucially, this movement is driven by the difference in water potential between the two regions. Water potential is a measure of the potential energy of water, reflecting its tendency to move from one area to another. A higher water potential indicates a greater tendency for water to move out of that region.
Water Potential: A Deeper Dive
Several factors contribute to the overall water potential. These include:
- Pressure potential: The pressure exerted on the water, such as turgor pressure within a plant cell.
- Solute potential (osmotic potential): The reduction in water potential due to the presence of solutes. A higher solute concentration results in a lower water potential. This is the primary driving force behind osmosis.
- Gravitational potential: The potential energy of water due to its position in a gravitational field, generally negligible in most biological systems.
Types of Osmosis: Hypotonic, Hypertonic, and Isotonic Solutions
The behavior of cells placed in solutions of varying solute concentrations helps illustrate the different types of osmosis:
1. Hypotonic Solutions: Swelling and Potential Lysis
A hypotonic solution has a lower solute concentration than the solution inside the cell. This means the water potential outside the cell is higher. Consequently, water moves into the cell across the membrane. This influx of water causes the cell to swell. In animal cells, this can lead to lysis, where the cell bursts due to excessive swelling. Plant cells, however, are protected by their rigid cell walls. The cell wall prevents the cell from bursting, resulting in turgor pressure, which gives the plant its rigidity and structure. This turgor pressure is vital for maintaining plant cell shape and function.
2. Hypertonic Solutions: Shrinking and Plasmolysis
A hypertonic solution has a higher solute concentration than the solution inside the cell. This means the water potential outside the cell is lower. Therefore, water moves out of the cell across the membrane, causing the cell to shrink. In animal cells, this process is called crenation. Plant cells undergo plasmolysis, where the cell membrane pulls away from the cell wall due to water loss. This process can severely impact the cell's function and survival.
3. Isotonic Solutions: Equilibrium and Stability
An isotonic solution has the same solute concentration as the solution inside the cell. This means the water potential is equal on both sides of the membrane. There is no net movement of water across the membrane, and the cell maintains its size and shape. This is generally the optimal condition for animal cells, while plant cells prefer a slightly hypotonic environment to maintain turgor pressure.
Osmosis in Biological Systems: Examples and Significance
Osmosis plays a critical role in countless biological processes:
1. Water Absorption in Plants: The Role of Root Hairs
Plants absorb water from the soil through their root hairs. The soil solution is typically hypotonic to the cells of the root hairs, causing water to move into the roots by osmosis. This water is then transported throughout the plant, ensuring hydration and nutrient delivery. The process is facilitated by the Casparian strip, a waterproof band in the root endodermis that directs water movement into the xylem, the plant's water-transporting tissue.
2. Maintaining Blood Pressure and Fluid Balance in Animals: The Kidneys and Osmosis
The kidneys play a vital role in regulating blood pressure and maintaining fluid balance. They do this partly through osmoregulation, a process that relies heavily on osmosis. The kidneys adjust the concentration of solutes in the urine, which influences water reabsorption back into the bloodstream. This fine-tuned control prevents dehydration or overhydration, maintaining a stable internal environment.
3. Nutrient Transport and Cell Signaling: Osmosis and Membrane Transport
Osmosis plays a critical role in nutrient transport across cell membranes. The movement of water influences the overall concentration gradients, impacting the transport of other solutes. For example, the process of facilitated diffusion and active transport, which require specific membrane proteins, are influenced by the osmotic environment. Water movement across membranes also plays a role in cell signaling processes, where changes in cell volume or turgor pressure can trigger signaling cascades.
Applications of Osmosis: Beyond Biology
The principles of osmosis have diverse applications beyond biological systems:
1. Water Purification: Reverse Osmosis
Reverse osmosis (RO) is a water purification technique that uses pressure to force water across a semipermeable membrane against the osmotic gradient. This effectively removes impurities, producing clean, drinkable water. RO systems are commonly used in homes and industries for water treatment.
2. Food Preservation: Osmotic Pressure
Osmotic pressure is used in food preservation techniques like pickling and salting. The high salt or sugar concentration in these solutions draws water out of microorganisms, inhibiting their growth and preserving the food.
3. Medical Applications: Osmosis and Dialysis
Osmosis is a fundamental principle behind dialysis, a treatment for kidney failure. Dialysis uses semipermeable membranes to remove waste products and excess water from the blood, effectively mimicking the function of healthy kidneys.
Conclusion: Osmosis—A Fundamental Life Process
Osmosis, the movement of water across a semipermeable membrane, is a fundamental process in biological systems and has far-reaching applications in various fields. Understanding the principles of osmosis, including water potential, semipermeable membranes, and the effects of different solute concentrations, is essential for grasping the intricacies of cellular function and its impact on life at all levels. From the smallest single-celled organism to the largest multicellular plants and animals, the ubiquitous nature of osmosis highlights its fundamental importance in maintaining life and function. Its influence extends beyond biology, with applications in water purification, food preservation, and medical treatments. Continued research into osmosis and its intricacies promises further advancements and insights into this critical life process.
Latest Posts
Latest Posts
-
How Many Hours Are In 210 Minutes
Apr 02, 2025
-
Does A Gas Have A Definite Volume
Apr 02, 2025
-
Which Base Is Found Only In Rna
Apr 02, 2025
-
Difference Between Meiosis 1 And Meiosis 2
Apr 02, 2025
-
Equation Of Circle In Parametric Form
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about The Movement Of Water Across A Semipermeable Membrane Is Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
