What Is The Gram Formula Mass Of Ca3 Po4 2
Juapaving
Mar 14, 2025 · 5 min read
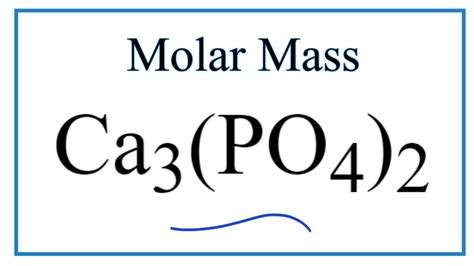
Table of Contents
What is the Gram Formula Mass of Ca₃(PO₄)₂? A Comprehensive Guide
Determining the gram formula mass (also known as molar mass or molecular weight) of a compound is a fundamental skill in chemistry. This article will provide a detailed explanation of how to calculate the gram formula mass of calcium phosphate, Ca₃(PO₄)₂, and delve into the concepts behind it. We'll also explore the significance of gram formula mass in various chemical calculations and applications.
Understanding Gram Formula Mass
The gram formula mass of a compound represents the mass of one mole of that substance, expressed in grams. One mole is a unit of measurement in chemistry that contains Avogadro's number (approximately 6.022 x 10²³) of entities, whether those are atoms, molecules, ions, or formula units. The gram formula mass is essentially the sum of the atomic masses of all the atoms present in the chemical formula, taking into account the number of atoms of each element.
Key Concepts:
- Atomic Mass: The average mass of an atom of an element, taking into account the relative abundance of its isotopes. You'll find these values on the periodic table.
- Mole (mol): A fundamental unit in chemistry, representing Avogadro's number of particles.
- Formula Unit: The smallest electrically neutral unit of an ionic compound. In the case of Ca₃(PO₄)₂, it's one unit of the compound consisting of 3 calcium ions and 2 phosphate ions.
- Molar Mass: Another term used interchangeably with gram formula mass.
Calculating the Gram Formula Mass of Ca₃(PO₄)₂
To calculate the gram formula mass of Ca₃(PO₄)₂, we need to consider the atomic masses of calcium (Ca), phosphorus (P), and oxygen (O). These values can be found on a periodic table. Let's assume the following atomic masses for our calculation (these values may vary slightly depending on the periodic table used):
- Ca (Calcium): 40.08 g/mol
- P (Phosphorus): 30.97 g/mol
- O (Oxygen): 16.00 g/mol
Now, let's break down the calculation step-by-step:
-
Calcium (Ca): There are 3 calcium atoms in the formula unit Ca₃(PO₄)₂. Therefore, the contribution from calcium is: 3 atoms × 40.08 g/mol/atom = 120.24 g/mol
-
Phosphorus (P): There are 2 phosphorus atoms in the formula unit (within the phosphate ion, PO₄). The contribution from phosphorus is: 2 atoms × 30.97 g/mol/atom = 61.94 g/mol
-
Oxygen (O): There are 8 oxygen atoms in the formula unit (4 oxygen atoms per phosphate ion, and 2 phosphate ions). The contribution from oxygen is: 8 atoms × 16.00 g/mol/atom = 128.00 g/mol
-
Total Gram Formula Mass: Finally, we sum up the contributions from each element: 120.24 g/mol + 61.94 g/mol + 128.00 g/mol = 310.18 g/mol
Therefore, the gram formula mass of Ca₃(PO₄)₂ is approximately 310.18 g/mol.
Significance of Gram Formula Mass
The gram formula mass is a crucial value in various chemical calculations and applications:
1. Mole Conversions:
Knowing the gram formula mass allows for easy conversions between grams and moles. This is essential for stoichiometric calculations, which involve determining the amounts of reactants and products in chemical reactions.
Example: If you have 10 grams of Ca₃(PO₄)₂, you can calculate the number of moles using the gram formula mass:
Moles = (mass in grams) / (gram formula mass) = 10 g / 310.18 g/mol ≈ 0.032 moles
2. Determining Empirical and Molecular Formulas:
The gram formula mass plays a significant role in determining the empirical and molecular formulas of compounds. The empirical formula represents the simplest whole-number ratio of atoms in a compound, while the molecular formula represents the actual number of atoms of each element in a molecule.
3. Solution Concentration Calculations:
Gram formula mass is used to calculate the molarity (moles of solute per liter of solution) and other concentration units of solutions. This is crucial in many chemical and biological applications.
4. Stoichiometry and Chemical Reactions:
Stoichiometric calculations rely heavily on gram formula mass to determine the amounts of reactants and products involved in chemical reactions. It allows you to predict the yield of a reaction or determine the limiting reactant.
5. Titrations:
In titrations, where a solution of known concentration is used to determine the concentration of an unknown solution, the gram formula mass is essential for accurately calculating the unknown concentration.
Common Mistakes to Avoid
When calculating gram formula mass:
- Pay close attention to subscripts: Ensure you correctly account for the number of atoms of each element in the formula. Subscripts outside parentheses apply to all elements within the parentheses.
- Use the correct atomic masses: Refer to a reliable periodic table for the most accurate atomic masses. Slight variations in atomic mass values exist depending on the table used.
- Double-check your calculations: It’s easy to make minor arithmetic errors, so always double-check your work to ensure accuracy.
Applications of Ca₃(PO₄)₂ and its Gram Formula Mass
Calcium phosphate, Ca₃(PO₄)₂, is a vital compound with numerous applications:
-
Fertilizers: It's a major component of many fertilizers, providing phosphorus, an essential nutrient for plant growth. The gram formula mass helps determine the amount of phosphorus present in a fertilizer sample.
-
Dietary Supplements: Calcium phosphate is used as a calcium supplement in various dietary products to support bone health. Understanding the gram formula mass is important for determining the calcium content per dosage.
-
Dental and Bone Implants: Its biocompatibility makes it useful in dental and bone implant materials. The gram formula mass is relevant in material science calculations for these applications.
-
Food Additives: It acts as a leavening agent and a firming agent in food products.
-
Industrial Applications: It finds use in various industrial processes, including water treatment and the production of ceramics.
Conclusion
Calculating the gram formula mass of Ca₃(PO₄)₂ is a straightforward process that involves summing the atomic masses of all atoms in the chemical formula. However, understanding the underlying concepts and the significance of this value in various chemical calculations is crucial for success in chemistry. The ability to accurately determine and utilize the gram formula mass is essential for many applications, ranging from stoichiometry and solution preparation to understanding the composition and properties of important compounds like calcium phosphate. The application of this knowledge extends beyond theoretical calculations to practical applications in fields like agriculture, medicine, and industry. Mastering this concept lays a strong foundation for more advanced chemical studies and practical applications.
Latest Posts
Latest Posts
-
The Terminal Electron Acceptor In Aerobic Respiration Is
Mar 14, 2025
-
Whats The Difference Between Alternator And Generator
Mar 14, 2025
-
What Does Xlv Mean In Roman Numbers
Mar 14, 2025
-
Is Melting Ice Chemical Or Physical Change
Mar 14, 2025
-
How Many Valence Electrons Are In Strontium
Mar 14, 2025
Related Post
Thank you for visiting our website which covers about What Is The Gram Formula Mass Of Ca3 Po4 2 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
