The Terminal Electron Acceptor In Aerobic Respiration Is
Juapaving
Mar 14, 2025 · 6 min read
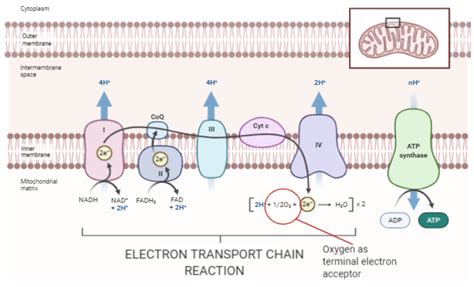
Table of Contents
The Terminal Electron Acceptor in Aerobic Respiration Is… Oxygen!
Aerobic respiration, the process that powers most life on Earth, hinges on a crucial component: the terminal electron acceptor. Understanding this component is key to grasping the intricacies of cellular respiration and its vital role in energy production. This article delves deep into the function and significance of oxygen as the terminal electron acceptor in aerobic respiration, exploring its chemical interactions, the consequences of its absence, and the broader implications for biological systems.
Understanding Cellular Respiration: A Recap
Before diving into the specifics of the terminal electron acceptor, let's briefly review the core process of cellular respiration. This metabolic pathway is responsible for converting the chemical energy stored in glucose and other organic molecules into a readily usable form of energy: ATP (adenosine triphosphate). Cellular respiration occurs in three main stages:
1. Glycolysis: The Initial Breakdown
Glycolysis takes place in the cytoplasm and involves the initial breakdown of glucose into two pyruvate molecules. This process generates a small amount of ATP and NADH, a crucial electron carrier.
2. The Krebs Cycle (Citric Acid Cycle): Harvesting Energy
Pyruvate enters the mitochondria, where it's converted into acetyl-CoA and enters the Krebs cycle. This cycle further breaks down acetyl-CoA, releasing carbon dioxide and generating more ATP, NADH, and FADH2 (another electron carrier).
3. Oxidative Phosphorylation: The Electron Transport Chain and Chemiosmosis
This is where the terminal electron acceptor comes into play. The NADH and FADH2 generated in glycolysis and the Krebs cycle carry high-energy electrons to the electron transport chain (ETC), located in the inner mitochondrial membrane. The ETC is a series of protein complexes that pass electrons down an energy gradient, releasing energy along the way. This energy is used to pump protons (H+) across the inner mitochondrial membrane, creating a proton gradient. The flow of protons back across the membrane, through ATP synthase, drives the synthesis of ATP—the primary energy currency of the cell.
Oxygen: The Final Electron Acceptor
The crucial aspect of this process is the terminal electron acceptor, the molecule that accepts the electrons at the end of the electron transport chain. In aerobic respiration, this terminal electron acceptor is oxygen (O2). Without oxygen, the electron transport chain would become blocked, halting ATP production.
Oxygen's high electronegativity makes it an ideal terminal electron acceptor. This means it has a strong tendency to attract electrons. As electrons travel down the ETC, they lose energy. Oxygen, at the end of the chain, readily accepts these low-energy electrons and combines with protons (H+) to form water (H2O). This reaction is crucial because it prevents the buildup of electrons in the ETC, ensuring the continuous flow of electrons and ATP production.
The Reaction:
4e⁻ + 4H⁺ + O₂ → 2H₂O
This seemingly simple reaction is the culmination of a complex series of redox reactions, vital for life as we know it. The reduction of oxygen to water is the final step in the electron transport chain and the defining feature of aerobic respiration.
The Significance of Oxygen as the Terminal Electron Acceptor
The role of oxygen is not simply to accept electrons; it’s the cornerstone of efficient energy production. Here’s why:
-
High ATP Yield: Aerobic respiration, with oxygen as the terminal electron acceptor, yields a significantly higher amount of ATP compared to anaerobic respiration. This high energy output is essential for the metabolic demands of complex organisms.
-
Efficient Electron Flow: Oxygen's high electronegativity ensures a continuous and efficient flow of electrons through the electron transport chain, maximizing ATP synthesis.
-
Waste Product Removal: The production of water as a byproduct is beneficial, as it's a harmless and readily eliminated waste product. Compare this to the waste products of anaerobic respiration, such as lactic acid or ethanol, which can be toxic at high concentrations.
-
Evolutionary Significance: The evolution of oxygenic photosynthesis, which released oxygen into the atmosphere, paved the way for the evolution of aerobic respiration. This shift to aerobic respiration significantly increased the energy available to organisms, driving the evolution of more complex life forms.
Consequences of Oxygen Absence: Anaerobic Respiration and Fermentation
When oxygen is unavailable, organisms must resort to alternative methods of energy production. These methods, collectively known as anaerobic respiration and fermentation, involve different terminal electron acceptors and yield significantly less ATP.
Anaerobic Respiration
In anaerobic respiration, other molecules, such as sulfate (SO₄²⁻), nitrate (NO₃⁻), or carbon dioxide (CO₂), serve as terminal electron acceptors. These processes are less efficient than aerobic respiration because the electron acceptors have lower electronegativity than oxygen, resulting in a smaller energy release during electron transfer. Anaerobic respiration is common in certain bacteria and archaea that thrive in oxygen-deprived environments.
Fermentation
Fermentation is another anaerobic pathway that doesn't involve an electron transport chain. Instead, it relies on substrate-level phosphorylation to generate a small amount of ATP. Fermentation regenerates NAD⁺ from NADH, allowing glycolysis to continue. Different types of fermentation exist, producing various byproducts, such as lactic acid (in humans during strenuous exercise) or ethanol (in yeast during alcoholic fermentation).
Exploring the Molecular Details: The Electron Transport Chain Complexes
The electron transport chain comprises four major protein complexes (Complexes I-IV) embedded in the inner mitochondrial membrane. These complexes facilitate the stepwise transfer of electrons, coupled with proton pumping.
- Complex I (NADH dehydrogenase): Receives electrons from NADH and transfers them to ubiquinone (CoQ).
- Complex II (succinate dehydrogenase): Receives electrons from FADH2 and also transfers them to CoQ.
- Complex III (cytochrome bc₁ complex): Transfers electrons from CoQ to cytochrome c.
- Complex IV (cytochrome c oxidase): The final complex, receiving electrons from cytochrome c and transferring them to oxygen, reducing it to water.
Each complex facilitates a specific step in the electron transfer process, contributing to the overall proton gradient that drives ATP synthesis. The precise mechanisms involving prosthetic groups like iron-sulfur clusters, heme groups, and copper ions within these complexes are intricate and fascinating areas of ongoing biochemical research. Understanding these details is fundamental to appreciating the elegance and efficiency of aerobic respiration.
The Interplay of Oxygen and Reactive Oxygen Species (ROS)
While oxygen is crucial for aerobic respiration, it also poses a potential threat. During the reduction of oxygen to water, some partially reduced oxygen species, known as reactive oxygen species (ROS), can be formed. ROS, including superoxide radicals (O₂⁻) and hydrogen peroxide (H₂O₂), are highly reactive and can damage cellular components like DNA, proteins, and lipids, leading to oxidative stress.
To combat the harmful effects of ROS, cells possess various antioxidant defense mechanisms, including enzymes such as superoxide dismutase (SOD) and catalase, which neutralize ROS. The balance between ROS production and antioxidant defense is crucial for maintaining cellular health and preventing oxidative damage. Dysfunction in this balance is implicated in various diseases, emphasizing the delicate relationship between oxygen and cellular well-being.
Conclusion: Oxygen—The Engine of Aerobic Life
In conclusion, oxygen's role as the terminal electron acceptor in aerobic respiration is paramount. Its high electronegativity enables efficient electron flow through the electron transport chain, leading to a high ATP yield—the energy currency that fuels life’s processes. The absence of oxygen necessitates alternative, less efficient energy-producing pathways. Understanding the intricacies of oxygen's role in cellular respiration, the consequences of its absence, and the interplay with ROS is fundamental to appreciating the complexity and elegance of life's fundamental processes. Ongoing research continues to unveil the subtleties of this crucial biochemical pathway, highlighting its profound impact on the diversity and evolution of life on Earth. The efficiency of oxygen as the terminal electron acceptor is not just a biochemical detail; it's the engine that drives the vibrant and diverse ecosystems we observe today.
Latest Posts
Latest Posts
-
What Is The Difference Between Open And Closed Circulatory Systems
Mar 14, 2025
-
10 Is A Multiple Of 5
Mar 14, 2025
-
How Many Edges Are There In A Rectangular Prism
Mar 14, 2025
-
Common Factors Of 32 And 40
Mar 14, 2025
-
Genes Had Been Absent On The Chromosomes
Mar 14, 2025
Related Post
Thank you for visiting our website which covers about The Terminal Electron Acceptor In Aerobic Respiration Is . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
