How Many Valence Electrons Are In Strontium
Juapaving
Mar 14, 2025 · 5 min read
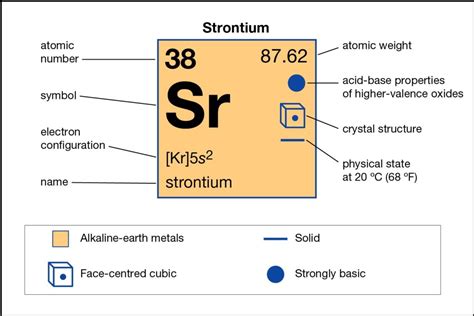
Table of Contents
How Many Valence Electrons are in Strontium? A Deep Dive into Atomic Structure
Strontium, a silvery-white alkaline earth metal, holds a fascinating place in the periodic table. Understanding its properties, particularly its number of valence electrons, is key to comprehending its chemical behavior and reactivity. This article will delve deep into the atomic structure of strontium, explaining precisely how many valence electrons it possesses and why this number is crucial to its characteristics. We will also explore related concepts such as electron configuration, the significance of valence electrons in chemical bonding, and strontium's applications.
Understanding Valence Electrons: The Key to Reactivity
Before we pinpoint the number of valence electrons in strontium, let's establish a foundational understanding of what valence electrons are. Valence electrons are the electrons located in the outermost shell (also known as the valence shell) of an atom. These electrons are the furthest from the nucleus and are therefore the most loosely bound. This loose binding makes them the primary players in chemical reactions. They are responsible for an atom's ability to form chemical bonds with other atoms. The number of valence electrons determines an element's chemical properties and how it will interact with other elements.
Strontium's Position in the Periodic Table: A Clue to its Valence Electrons
The periodic table is organized in a way that provides valuable information about the elements. Strontium (Sr) is located in Group 2, also known as the alkaline earth metals. This group's defining characteristic is having two valence electrons. This is a fundamental rule: the group number (for Groups 1-2 and 13-18) directly correlates to the number of valence electrons for the main group elements.
Electron Configuration: Unveiling the Strontium Atom's Structure
To further solidify our understanding, let's examine strontium's electron configuration. The electron configuration describes how electrons are distributed among the various energy levels and sublevels within an atom. Strontium's atomic number is 38, meaning it has 38 electrons. Its electron configuration is: 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁶5s².
Notice the last two electrons are in the 5s orbital. This outermost shell (n=5) contains these two electrons, confirming our earlier deduction from its group position. Therefore, strontium unequivocally possesses two valence electrons.
The Significance of Two Valence Electrons: Chemical Behavior of Strontium
The presence of two valence electrons profoundly influences strontium's chemical behavior. Alkaline earth metals like strontium are highly reactive, readily losing their two valence electrons to achieve a stable, filled electron shell configuration like that of the noble gas krypton. This process of losing electrons is called oxidation.
This eagerness to lose electrons makes strontium a strong reducing agent. It readily reacts with many elements, particularly nonmetals like oxygen, halogens (chlorine, bromine, iodine), and sulfur, forming ionic compounds. For instance, strontium reacts vigorously with oxygen to form strontium oxide (SrO).
Chemical Bonding: How Strontium Forms Compounds
Strontium's two valence electrons play a crucial role in its ability to form chemical bonds. It typically forms ionic bonds, where it loses its two valence electrons to become a positively charged ion (Sr²⁺). These electrons are then accepted by another atom, usually a nonmetal, creating a negatively charged ion. The electrostatic attraction between the positively and negatively charged ions forms the ionic bond.
Applications of Strontium: Leveraging its Properties
The unique properties of strontium, directly linked to its two valence electrons and its reactivity, lead to diverse applications across various industries:
1. Pyrotechnics: Creating Vibrant Red Colors
Strontium salts are widely used in fireworks to produce a brilliant red color. The excited strontium ions emit light at a specific wavelength within the red region of the visible spectrum.
2. Metallurgy: Alloying Agent
Strontium is employed as an alloying agent in various metals, improving their properties like strength, ductility, and machinability.
3. Medical Applications: Diagnosing and Treating Diseases
Certain strontium isotopes are used in medical imaging and treatment. For example, strontium-89 is used in palliative radiotherapy to treat bone metastases in cancer patients.
4. Ceramics and Glass: Enhancing Properties
Strontium compounds are added to ceramics and glass to modify their properties, enhancing their strength, durability, and optical characteristics.
5. Electronic Applications: Specialized Devices
Strontium titanate, a strontium compound, possesses unique electronic properties, making it useful in some electronic devices.
Beyond the Valence Electrons: A Deeper Look at Strontium's Properties
While the number of valence electrons is crucial for understanding strontium's basic chemical reactivity, it's important to note that other factors contribute to its overall properties. These include:
- Atomic size: Strontium's relatively large atomic size influences its bond strength and reactivity.
- Ionization energy: The energy required to remove an electron from a strontium atom affects its tendency to form ions.
- Electronegativity: Strontium's low electronegativity indicates its tendency to lose electrons rather than gain them.
Conclusion: The Importance of Valence Electrons in Understanding Strontium
In conclusion, strontium possesses two valence electrons. This seemingly simple fact is the key to unlocking its chemical behavior, reactivity, and ultimately, its numerous applications. The number of valence electrons directly dictates its ability to form ionic bonds, its reducing nature, and its role in various technological advancements. Understanding the fundamental concept of valence electrons is crucial to appreciating the complexities of chemical interactions and the properties of elements across the periodic table. This comprehensive exploration showcases the importance of this seemingly small detail in understanding the fascinating world of chemistry. Further research into strontium and other alkaline earth metals will further illuminate the deeper nuances of chemical bonding and reactivity within the context of their unique electron configurations.
Latest Posts
Latest Posts
-
Common Factors Of 32 And 40
Mar 14, 2025
-
Genes Had Been Absent On The Chromosomes
Mar 14, 2025
-
What Is The Subunit For Lipids
Mar 14, 2025
-
Why Is The Earth Called Blue Planet
Mar 14, 2025
-
Least Common Multiple Of 21 And 28
Mar 14, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Are In Strontium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
