What Is The Formula Of Zinc Chloride
Juapaving
Mar 22, 2025 · 6 min read
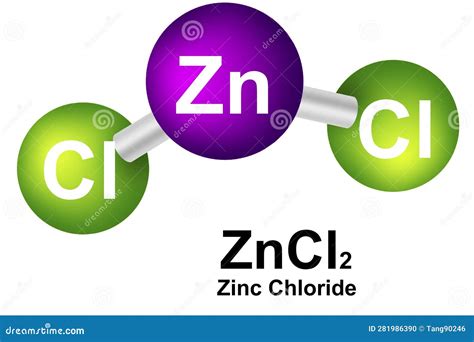
Table of Contents
What is the Formula of Zinc Chloride? A Deep Dive into its Chemistry, Properties, and Applications
Zinc chloride, a ubiquitous chemical compound, finds extensive use across diverse fields. Understanding its chemical formula is fundamental to comprehending its properties and applications. This comprehensive article delves into the intricacies of zinc chloride, exploring its formula, properties, synthesis methods, safety precautions, and diverse applications.
Understanding the Chemical Formula: ZnCl₂
The chemical formula of zinc chloride is ZnCl₂. This simple yet powerful formula reveals the fundamental composition of the compound:
- Zn: Represents one atom of zinc (a transition metal). Zinc, with its atomic number 30, readily loses two electrons to achieve a stable electron configuration.
- Cl₂: Represents two atoms of chlorine (a halogen). Chlorine, with atomic number 17, readily gains one electron to complete its outermost electron shell.
The formula ZnCl₂ indicates that one zinc atom bonds ionically with two chlorine atoms. The zinc atom loses two electrons, forming a Zn²⁺ cation (positively charged ion), and each chlorine atom gains one electron, forming two Cl⁻ anions (negatively charged ions). The electrostatic attraction between the positively charged zinc ion and the negatively charged chloride ions results in the formation of the ionic compound zinc chloride.
Properties of Zinc Chloride
Zinc chloride exhibits several key properties that contribute to its wide range of applications:
Physical Properties:
- Appearance: It typically appears as a white, crystalline powder, although it can also exist in a granular or fused form.
- Melting Point: Relatively low melting point of 290 °C (554 °F).
- Boiling Point: High boiling point of 732 °C (1350 °F).
- Solubility: Highly soluble in water, exhibiting significant exothermic behavior (heat is released upon dissolution). It's also soluble in many organic solvents like ethanol and ether.
- Hygroscopic Nature: ZnCl₂ is highly hygroscopic, meaning it readily absorbs moisture from the air, often deliquescing (becoming liquid) in humid conditions. This property necessitates careful storage in airtight containers to prevent its degradation.
- Density: The density of zinc chloride varies depending on its form, but it is generally relatively dense.
Chemical Properties:
- Ionic Nature: As an ionic compound, zinc chloride readily dissociates into its constituent ions (Zn²⁺ and Cl⁻) when dissolved in water. This property allows it to participate in various chemical reactions.
- Lewis Acidity: Zinc chloride acts as a Lewis acid, meaning it can accept an electron pair from a Lewis base. This property is crucial in its catalytic activity in many chemical reactions.
- Reactivity with Water: Its reaction with water is exothermic, producing heat. This reaction can be significant and needs to be considered during handling and use.
- Reactivity with Bases: Zinc chloride reacts with bases to form zinc hydroxide and the corresponding salt.
- Complex Formation: Zinc chloride readily forms complexes with various ligands, further expanding its applications in coordination chemistry.
Synthesis of Zinc Chloride
Zinc chloride can be synthesized through several methods:
Reaction of Zinc Metal with Hydrochloric Acid:
This is a common and straightforward method:
Zn(s) + 2HCl(aq) → ZnCl₂(aq) + H₂(g)
Zinc metal reacts with hydrochloric acid to produce zinc chloride and hydrogen gas. The reaction is exothermic and produces heat. The resulting solution can be evaporated to obtain crystalline zinc chloride.
Reaction of Zinc Oxide with Hydrochloric Acid:
Zinc oxide also reacts with hydrochloric acid to produce zinc chloride and water:
ZnO(s) + 2HCl(aq) → ZnCl₂(aq) + H₂O(l)
This method is also relatively simple and provides a high yield of zinc chloride.
Direct Chlorination of Zinc Metal:
Zinc metal can be directly chlorinated by passing chlorine gas over heated zinc metal:
Zn(s) + Cl₂(g) → ZnCl₂(s)
This method requires careful control of the reaction conditions to avoid the formation of unwanted byproducts.
Safety Precautions when Handling Zinc Chloride
Zinc chloride is generally considered to be a relatively safe compound, but appropriate safety measures are essential during handling and usage:
- Eye Protection: Always wear appropriate safety goggles or face shields to protect your eyes from potential splashes or dust.
- Skin Protection: Wear gloves and appropriate clothing to prevent skin contact. Zinc chloride can be irritating to the skin.
- Respiratory Protection: In case of dust or fumes, a respirator is recommended to prevent inhalation.
- Ventilation: Ensure adequate ventilation in the area where zinc chloride is being handled to minimize exposure to fumes.
- Storage: Store zinc chloride in airtight containers in a cool, dry place to prevent its deliquescence and degradation.
- Disposal: Dispose of zinc chloride according to local regulations and guidelines.
Applications of Zinc Chloride
The versatility of zinc chloride is reflected in its wide range of applications across various industries:
Industrial Applications:
- Metal Working: Used as a flux in soldering and welding, improving the flow of molten metal and cleaning metal surfaces.
- Wood Preservation: Employed as a wood preservative, offering protection against decay and insect infestation.
- Textile Industry: Used as a mordant in dyeing and printing fabrics, enhancing the color fastness and improving the dye's adherence to the fibers.
- Catalysis: Acts as a catalyst in various chemical reactions, particularly in organic synthesis.
- Electroplating: Used in electroplating processes to enhance the deposition of zinc on other metals.
- Concrete: Can be added to concrete to improve its properties, such as strength and setting time.
Medical Applications:
- Antiseptic: Zinc chloride possesses antiseptic properties and has been used in some antiseptic solutions for wound treatment. However, its current use in this capacity is limited due to the availability of more effective and less irritating antiseptic agents.
- Medicine: While not a commonly used pharmaceutical, it has had limited application in certain specialized medicinal preparations.
Other Applications:
- Dehydrating Agent: Zinc chloride's strong affinity for water makes it a useful dehydrating agent in certain chemical processes.
- Laboratory Reagent: Widely used in analytical chemistry and as a reagent in various chemical reactions.
- Agriculture: Limited use in agriculture as a micronutrient supplement.
Conclusion: A Versatile Compound with Broader Implications
Zinc chloride, with its simple formula ZnCl₂, showcases remarkable versatility. Its unique chemical and physical properties have led to its widespread adoption across diverse sectors, ranging from industrial manufacturing to specialized medicinal preparations. Understanding its formula, properties, synthesis, and safety considerations is crucial for responsible handling and harnessing its potential in numerous applications. Further research and development may unlock even more applications for this remarkable chemical compound. The ongoing exploration of zinc chloride's potential emphasizes its importance in both established and emerging technologies. While its direct use in consumer products is less common than other zinc compounds, its role as a critical ingredient and intermediate in countless manufacturing processes ensures its continued significance in the modern world. This inherent versatility positions zinc chloride as a key player in advancing both chemical and engineering sciences.
Latest Posts
Latest Posts
-
Fog Is An Example Of A
Mar 23, 2025
-
What Is The Similarity Between A Compound And A Mixture
Mar 23, 2025
-
How To Spell 18 In Words
Mar 23, 2025
-
Kelvin Planck Second Law Of Thermodynamics
Mar 23, 2025
-
Are There Mitochondria In Plant Cells
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about What Is The Formula Of Zinc Chloride . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
