Kelvin Planck Second Law Of Thermodynamics
Juapaving
Mar 23, 2025 · 6 min read
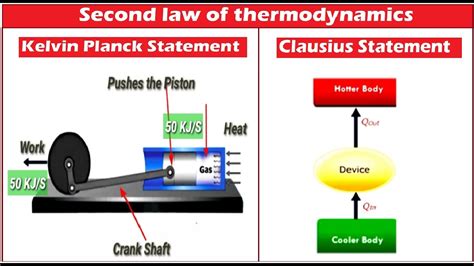
Table of Contents
Kelvin-Planck Statement of the Second Law of Thermodynamics: A Deep Dive
The Second Law of Thermodynamics is a cornerstone of physics, dictating the directionality of natural processes and setting fundamental limits on energy conversion. While there are several equivalent formulations, the Kelvin-Planck statement offers a particularly intuitive understanding, focusing on the impossibility of creating a perpetual motion machine of the second kind. This article will delve deep into the Kelvin-Planck statement, exploring its implications, applications, and connections to other thermodynamic concepts.
Understanding the Kelvin-Planck Statement
The Kelvin-Planck statement, also known as the Lord Kelvin statement, asserts: It is impossible to devise a cyclically operating device, the sole effect of which is to absorb energy in the form of heat from a single thermal reservoir and deliver an equivalent amount of work.
Let's break this down:
-
Cyclically operating device: This means the device undergoes a series of processes that eventually return it to its initial state. This is crucial because it eliminates the possibility of simply using up a reservoir of energy in a one-time process. The focus is on continuous operation.
-
Sole effect: This is the key to understanding the impossibility. The statement isn't saying you can't extract work from a heat reservoir; it says you can't extract an equivalent amount of work without some other effect occurring.
-
Single thermal reservoir: The statement emphasizes that the device interacts with only one heat source. This eliminates scenarios where heat is transferred from a hotter reservoir to a colder reservoir, enabling work extraction (as in a heat engine).
-
Absorb energy in the form of heat: The energy input must be exclusively heat. This excludes other forms of energy input, such as mechanical work.
-
Deliver an equivalent amount of work: The amount of work produced must be equal to the heat absorbed. No energy is lost or gained. This eliminates any possibility of energy efficiency.
Essentially, the Kelvin-Planck statement postulates that you cannot build a heat engine that operates with 100% efficiency, extracting heat from a single reservoir and converting it entirely into work. Such a device would violate the second law.
The Implications of the Kelvin-Planck Statement
The Kelvin-Planck statement has profound implications for various aspects of thermodynamics and engineering:
1. Limits on Energy Conversion:
The statement establishes a fundamental limit on the efficiency of heat engines. No heat engine can convert all the heat it absorbs into useful work; some heat must always be rejected to a colder reservoir. This inherent inefficiency is a direct consequence of the second law.
2. Irreversibility of Processes:
The statement highlights the irreversibility of natural processes. The spontaneous conversion of heat entirely into work is impossible; the reverse process – the complete conversion of work into heat – is always possible. This asymmetry is a hallmark of the second law.
3. Defining Entropy:
The Kelvin-Planck statement, along with other formulations of the second law, provides a basis for the concept of entropy. Entropy is a measure of disorder or randomness in a system. The second law states that the total entropy of an isolated system can only increase over time, or remain constant in ideal cases where the system is in a steady state or undergoing a reversible process. The impossibility of perfectly converting heat to work is directly linked to the increase in entropy.
4. Designing Efficient Heat Engines:
Engineers use the Kelvin-Planck statement as a guideline to design more efficient heat engines. By understanding the limitations imposed by the second law, they can optimize engine designs to maximize the conversion of heat into work while minimizing heat rejection.
Comparing Kelvin-Planck with Clausius Statement
Another important statement of the second law is the Clausius statement, which says: It is impossible to devise a cyclically operating device, the sole effect of which is to transfer heat from a cooler body to a hotter body.
Both the Kelvin-Planck and Clausius statements are equivalent; proving one implies the other. The Kelvin-Planck statement focuses on the impossibility of creating a perfect heat engine, while the Clausius statement focuses on the impossibility of spontaneous heat transfer from cold to hot. This highlights the inherent directionality of heat flow.
Mathematical Representation and Carnot Efficiency
While the Kelvin-Planck statement is qualitative, its implications are quantifiable through the Carnot cycle and the concept of Carnot efficiency. The Carnot cycle represents a theoretical ideal heat engine operating reversibly between two thermal reservoirs at temperatures T<sub>H</sub> (hot) and T<sub>C</sub> (cold).
The Carnot efficiency (η<sub>C</sub>) is given by:
η<sub>C</sub> = 1 - (T<sub>C</sub> / T<sub>H</sub>)
Where temperatures are expressed in absolute units (Kelvin). This equation demonstrates that the efficiency of even an ideal reversible engine is always less than 1 (100%), confirming the Kelvin-Planck statement. The efficiency increases as the temperature difference between the reservoirs increases. Real-world engines always have efficiencies lower than the Carnot efficiency due to irreversibilities such as friction and heat losses.
Applications of Kelvin-Planck Statement
The Kelvin-Planck statement has broad applications in various fields:
1. Power Generation:
Power plants, whether they use fossil fuels, nuclear energy, or renewable sources, are fundamentally heat engines governed by the second law. The Kelvin-Planck statement dictates the limitations on their efficiency, driving research and development to improve designs and reduce energy waste.
2. Refrigeration and Air Conditioning:
Refrigeration systems work by transferring heat from a cold reservoir (inside a refrigerator) to a hot reservoir (the surroundings). These systems require work input, and the Kelvin-Planck statement helps in understanding the minimum work required for a given cooling effect.
3. Aerospace Engineering:
Rocket engines and jet engines are also governed by thermodynamic principles, and the Kelvin-Planck statement is essential for optimizing their performance and fuel efficiency.
4. Chemical Engineering:
In chemical processes, the second law guides the design of efficient separation and purification techniques. Understanding the limitations on energy conversion aids in minimizing energy consumption and maximizing process efficiency.
Beyond the Statement: Implications for Sustainability
The Kelvin-Planck statement is not merely an academic curiosity; it has profound implications for sustainability. The inherent inefficiency of energy conversion emphasizes the importance of:
-
Energy conservation: Reducing energy consumption through improved efficiency and responsible use is critical to mitigate the environmental impact of energy generation.
-
Renewable energy sources: Transitioning to renewable energy sources such as solar, wind, and geothermal energy can help reduce reliance on fossil fuels and lessen greenhouse gas emissions.
-
Developing advanced energy technologies: Ongoing research and development in energy technologies are crucial for improving energy conversion efficiency and creating more sustainable energy systems.
Conclusion: The Enduring Relevance of the Kelvin-Planck Statement
The Kelvin-Planck statement of the second law of thermodynamics is a cornerstone of physics, providing a profound understanding of the limitations on energy conversion. Its implications extend beyond theoretical physics, influencing engineering design, energy policy, and our pursuit of sustainability. By recognizing the fundamental constraints imposed by the second law, we can make informed decisions to create more efficient and environmentally responsible energy systems for the future. The principle remains as relevant today as it was when first formulated, shaping our understanding of the universe and our place within it. Further study into the complexities of entropy and its relationship to the Kelvin-Planck statement reveals a deeper appreciation for the universal laws governing energy and the limitations of our technological abilities. The quest for increased efficiency remains a driving force in scientific and engineering endeavors, highlighting the enduring significance of this fundamental principle.
Latest Posts
Latest Posts
-
Why Is The Lagging Strand Made Up Of Ozaukee Fragments
Mar 25, 2025
-
What Is The Lowest Common Multiple Of 10 And 12
Mar 25, 2025
-
Least Common Multiple Of 7 9
Mar 25, 2025
-
Is Cooking A Chemical Or Physical Change
Mar 25, 2025
-
Is Concave Mirror Converging Or Diverging
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Kelvin Planck Second Law Of Thermodynamics . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
