What Is The Conjugate Base Of Hf
Juapaving
Mar 25, 2025 · 5 min read
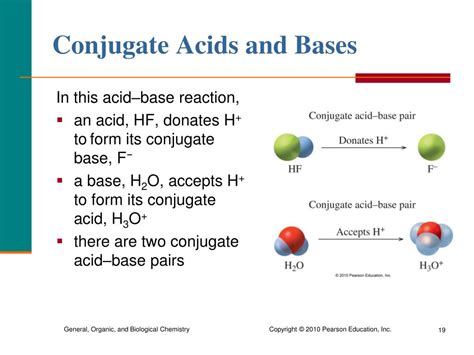
Table of Contents
What is the Conjugate Base of HF? A Deep Dive into Acid-Base Chemistry
Understanding conjugate acid-base pairs is fundamental to grasping acid-base chemistry. This article delves deep into the concept, focusing specifically on the conjugate base of hydrofluoric acid (HF), exploring its properties, reactions, and significance. We'll examine the broader context of Brønsted-Lowry acid-base theory, explore the unique characteristics of fluoride ion (F⁻), and discuss its applications.
Brønsted-Lowry Acid-Base Theory: The Foundation
Before we pinpoint the conjugate base of HF, let's establish a solid understanding of the Brønsted-Lowry theory. This theory defines an acid as a substance that donates a proton (H⁺), and a base as a substance that accepts a proton. Crucially, this theory emphasizes the transfer of protons in acid-base reactions.
When an acid donates a proton, it forms its conjugate base. Conversely, when a base accepts a proton, it forms its conjugate acid. This forms a conjugate acid-base pair, linked by the difference of a single proton.
Identifying the Conjugate Base of HF
Hydrofluoric acid (HF) is a weak acid, meaning it only partially dissociates in water. Its dissociation reaction is:
HF(aq) + H₂O(l) ⇌ H₃O⁺(aq) + F⁻(aq)
In this reaction, HF acts as a Brønsted-Lowry acid, donating a proton (H⁺) to a water molecule (H₂O). The water molecule acts as a Brønsted-Lowry base, accepting the proton.
The resulting products are the hydronium ion (H₃O⁺) – the conjugate acid of water – and the fluoride ion (F⁻) – the conjugate base of HF. The fluoride ion is the species remaining after HF has lost its proton.
Therefore, the conjugate base of HF is F⁻.
Properties of the Fluoride Ion (F⁻)
The fluoride ion, F⁻, is a negatively charged anion. Its properties are significantly influenced by its high electronegativity and small size. Let's explore some key characteristics:
1. High Electronegativity:
Fluorine is the most electronegative element on the periodic table. This means it strongly attracts electrons towards itself. This high electronegativity contributes to the fluoride ion's strong basicity, making it capable of accepting a proton.
2. Small Size and High Charge Density:
The small size of the fluoride ion results in a high charge density. This means the negative charge is concentrated in a small volume, leading to strong interactions with other ions and molecules. This contributes to its reactivity and solubility properties.
3. Weak Basicity:
While F⁻ is a base, it's considered a weak base. This is because HF is a relatively strong acid. The stronger an acid, the weaker its conjugate base, and vice versa. This is reflected in the relatively small equilibrium constant for the reaction of F⁻ with water:
F⁻(aq) + H₂O(l) ⇌ HF(aq) + OH⁻(aq)
This reaction shows that the fluoride ion only partially reacts with water to produce hydroxide ions (OH⁻), indicative of its weak basicity.
4. Reactivity and Complex Formation:
The fluoride ion exhibits reactivity with various metal cations, forming complex ions. This is due to its ability to act as a ligand, donating a lone pair of electrons to form coordinate bonds with metal ions. This property is utilized in various applications, as we'll discuss later.
5. Solubility:
The solubility of fluoride salts varies depending on the cation they are paired with. Some fluoride salts are highly soluble in water, while others are relatively insoluble.
Reactions Involving the Fluoride Ion
The fluoride ion participates in several important reactions:
1. Reaction with Acids:
As a weak base, F⁻ reacts with acids, accepting a proton to reform HF. The extent of this reaction depends on the strength of the acid. Strong acids will effectively convert F⁻ back to HF.
2. Reaction with Water (Hydrolysis):
As mentioned previously, F⁻ undergoes hydrolysis in water, producing a small amount of OH⁻ ions. This slightly increases the pH of the solution.
3. Formation of Metal Fluoride Complexes:
The fluoride ion readily forms complexes with many metal ions. These complexes often exhibit unique properties and applications. For example, the hexafluorophosphate(V) ion, [PF₆]⁻, is a common counterion in various chemical compounds.
4. Reactions in Toothpaste:
Fluoride ions are commonly found in toothpaste due to their ability to strengthen tooth enamel. The fluoride ions replace hydroxide ions in the hydroxyapatite of teeth, forming a stronger fluorapatite, thereby increasing resistance to acid attacks from bacteria.
Applications of Fluoride and its Compounds
The fluoride ion and its compounds find widespread applications across various fields:
1. Dentistry:
As discussed above, fluoride is crucial for dental health, preventing tooth decay. It's incorporated into toothpastes, mouthwashes, and even water fluoridation programs to enhance dental health on a public level.
2. Industry:
Fluoride compounds are used in various industrial processes. For example, hydrogen fluoride (HF) is used in the production of refrigerants and other fluorocarbons. However, it's crucial to handle HF with extreme caution due to its toxicity and corrosive nature. Other fluoride compounds find applications in the manufacturing of ceramics, glass, and other materials.
3. Medicine:
Certain fluoride compounds have medicinal applications. Some are used as antiseptics, while others play a role in treating specific medical conditions. However, it is important to note that excessive fluoride intake can lead to health problems.
4. Nuclear Energy:
Fluoride compounds are used in the nuclear industry, often as solvents or in the processing of nuclear fuels. Uranium hexafluoride (UF₆) is a crucial compound in uranium enrichment processes.
The Significance of Understanding Conjugate Bases
Understanding the concept of conjugate bases is essential for several reasons:
- Predicting Reaction Outcomes: Knowing the conjugate base allows us to predict the outcome of acid-base reactions and equilibrium positions.
- Buffer Solutions: Buffer solutions, crucial in maintaining stable pH levels, typically comprise a weak acid and its conjugate base.
- Solubility and Complexation: The properties of conjugate bases heavily influence the solubility and complex formation of various compounds.
- Catalysis: Certain conjugate bases act as catalysts in various chemical reactions.
Conclusion: F⁻ – A Vital Anion
The fluoride ion, F⁻, the conjugate base of HF, is far from an unremarkable species. Its high electronegativity, small size, and resulting properties lead to a wide array of applications across various fields. Understanding its behavior, reactivity, and significance within the framework of Brønsted-Lowry theory is crucial for comprehending acid-base chemistry and various chemical processes. From strengthening tooth enamel to its industrial applications, the fluoride ion's impact is significant and pervasive. Further research continues to uncover new applications and refine our understanding of this vital anion.
Latest Posts
Latest Posts
-
98 As A Product Of Prime Factors
Mar 25, 2025
-
What Are Vertices Of A Triangle
Mar 25, 2025
-
Is Air A Good Heat Conductor
Mar 25, 2025
-
Name Of A Seven Sided Polygon
Mar 25, 2025
-
Drops Collected From In Column Chromatography
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about What Is The Conjugate Base Of Hf . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
