What Is A Row In A Periodic Table
Juapaving
Mar 30, 2025 · 6 min read
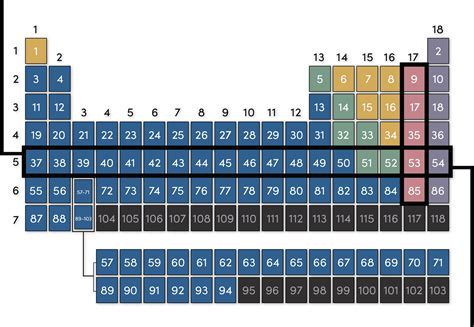
Table of Contents
What is a Row in a Periodic Table? Understanding Periods and Their Significance
The periodic table, a cornerstone of chemistry, organizes chemical elements in a structured grid, revealing patterns in their properties and behaviors. Understanding this organization is crucial for grasping chemical principles. While columns (groups) represent elements sharing similar chemical characteristics, rows (periods) depict a different, yet equally important, aspect: the gradual filling of electron shells. This article delves deep into what a row (or period) in the periodic table represents, exploring its significance and implications for understanding atomic structure and chemical reactivity.
Understanding the Structure: Periods and Electron Shells
A row in the periodic table, also known as a period, represents elements with the same number of electron shells. An electron shell, or energy level, is a region around the nucleus of an atom where electrons are likely to be found. These shells are labeled with integers (1, 2, 3, etc.), with shell 1 being closest to the nucleus and having the lowest energy. Each shell can accommodate a specific maximum number of electrons.
- Period 1: Contains only hydrogen (H) and helium (He), both having electrons in the first electron shell (n=1), which can hold a maximum of two electrons.
- Period 2: Includes elements like lithium (Li) to neon (Ne). These elements have electrons filling their second electron shell (n=2), capable of holding up to eight electrons.
- Period 3: Elements such as sodium (Na) to argon (Ar) have electrons populating their third electron shell (n=3), also with a maximum capacity of eight electrons.
- Period 4 onwards: The pattern continues, with each subsequent period adding another electron shell. However, the complexity increases, with the introduction of subshells (s, p, d, f) within each shell, leading to variations in electron configuration and properties.
The significance of electron shells and their relation to periods is paramount: The number of electron shells determines the atom's size and its reactivity. Elements within the same period show a gradual change in properties across the row due to the sequential addition of protons and electrons, affecting the effective nuclear charge and influencing electron behavior.
Periodic Trends Across a Period: A Gradual Shift in Properties
As we traverse a period from left to right, several key properties of elements exhibit predictable trends. These trends are directly linked to the increasing number of protons and electrons within the same electron shell.
1. Atomic Radius: A Decreasing Trend
Atomic radius, the distance from the nucleus to the outermost electron, generally decreases across a period. This is because the addition of protons increases the positive nuclear charge, pulling the electrons closer to the nucleus. While additional electrons are added, they are in the same shell, and the effect of the increased nuclear charge dominates, resulting in a smaller atomic size.
2. Ionization Energy: An Increasing Trend
Ionization energy is the energy required to remove an electron from a gaseous atom. This value generally increases across a period. The stronger nuclear pull on the electrons in the same shell makes it increasingly difficult to remove an electron, hence the higher ionization energy.
3. Electronegativity: A Generally Increasing Trend
Electronegativity measures an atom's ability to attract electrons in a chemical bond. It generally increases across a period, mirroring the trend in ionization energy. Atoms with higher nuclear charge attract shared electrons more strongly. However, some irregularities may occur due to the subtle interplay of electron-electron repulsions and effective nuclear charge.
4. Metallic Character: A Decreasing Trend
Metallic character, which relates to properties like conductivity and malleability, generally decreases across a period. Elements on the left side of a period are typically metals, while elements on the right are nonmetals. This transition reflects the increasing tendency to gain electrons (nonmetals) rather than lose them (metals) to achieve a stable electron configuration.
Beyond the Simple Trends: Exceptions and Subtleties
While the general trends described above are helpful, they are not absolute. There are exceptions and complexities that arise due to the nuances of electron configurations and electron-electron interactions. For instance:
- Electron-electron repulsion: The addition of electrons in the same shell leads to increased electron-electron repulsion, partially counteracting the effect of increased nuclear charge. This repulsion can slightly influence atomic radius and ionization energy.
- Shielding effect: Inner electrons can shield the outer electrons from the full effect of the nuclear charge, reducing the effective nuclear charge experienced by the outer electrons. This effect can influence the observed trends.
- Subshell effects: The different subshells (s, p, d, f) within a shell have slightly different energies, leading to variations in electron configurations and influencing the periodic trends. This is particularly noticeable in the later periods with the inclusion of d and f subshells.
The Importance of Periods in Understanding Chemical Reactivity
The position of an element within a period directly impacts its chemical reactivity. Elements on the far left (alkali metals) readily lose one electron to achieve a stable electron configuration, making them highly reactive. Conversely, elements on the far right (noble gases) have a stable electron configuration and are chemically inert. Elements in between exhibit varying degrees of reactivity, depending on their tendency to gain, lose, or share electrons.
The gradual change in properties across a period underlies the rich diversity of chemical behavior observed in nature. Understanding these trends allows us to predict and explain the formation of compounds, their properties, and their reactivity.
Periods and the Building-Up Principle (Aufbau Principle)
The organization of the periodic table is intrinsically linked to the Aufbau principle, which describes the sequential filling of atomic orbitals with electrons. As we move across a period, electrons are progressively added to the orbitals of the outermost electron shell, resulting in the characteristic trends observed in atomic properties. This principle helps explain why elements within the same period have similar electron shell structures but varying numbers of electrons in that shell.
The Extended Periodic Table and the Filling of f-orbitals
The later periods (6 and 7) exhibit a greater complexity due to the filling of the inner f-orbitals (lanthanides and actinides). These elements are often placed separately at the bottom of the table to maintain a manageable table structure. The filling of these f-orbitals introduces subtle variations in periodic trends, often leading to less pronounced changes in properties compared to the filling of s and p-orbitals.
Applications and Relevance
Understanding periods and the trends they represent has far-reaching implications across various fields:
- Material Science: Predicting and designing materials with specific properties. By understanding the relationship between atomic structure and properties, we can tailor materials for various applications.
- Chemical Synthesis: Designing and predicting the outcomes of chemical reactions based on the reactivity of elements within a period.
- Environmental Science: Understanding the behavior of elements in the environment and their impact on ecosystems.
- Medical Science: Understanding the role of elements in biological systems and their interactions with living organisms.
Conclusion: Periods as a Foundation for Chemical Understanding
In summary, a row (period) in the periodic table represents a crucial organizational principle reflecting the sequential filling of electron shells. This fundamental structure dictates periodic trends in atomic properties like atomic radius, ionization energy, electronegativity, and metallic character. Understanding these trends is essential for predicting and explaining chemical reactivity, compound formation, and the diverse properties of elements. The periodic table, with its organized rows and columns, provides a powerful framework for comprehending the vast and fascinating world of chemistry and its applications in numerous scientific disciplines. Further exploration of electron configurations, orbital theory, and the complexities of chemical bonding will only deepen this understanding, solidifying the importance of the periodic table's rows as a cornerstone of chemical knowledge.
Latest Posts
Latest Posts
-
Find The Inverse Of The Relation
Apr 01, 2025
-
How Are Cellular Respiration And Photosynthesis Related
Apr 01, 2025
-
How Many Rna Polymerases Are Found In Prokaryotes
Apr 01, 2025
-
When Two Parallel Lines Are Crossed By A Transversal
Apr 01, 2025
-
Device That Converts Light Energy Into Electrical Energy
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about What Is A Row In A Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
