What Are The Most Reactive Elements On The Periodic Table
Juapaving
Mar 17, 2025 · 6 min read
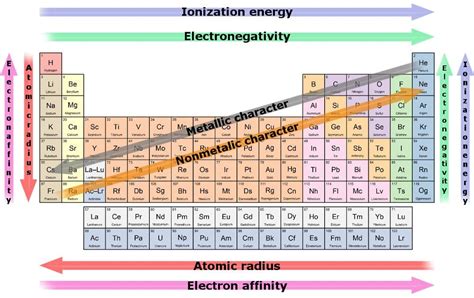
Table of Contents
What Are the Most Reactive Elements on the Periodic Table?
The periodic table, a beautifully organized chart of the elements, reveals much more than just atomic numbers and weights. It unveils the inherent properties of elements, including their reactivity—their tendency to undergo chemical reactions with other substances. Understanding reactivity is key to predicting how elements will behave and interact, crucial in fields ranging from materials science to medicine. This article dives deep into the most reactive elements, exploring their characteristics, reactions, and applications.
The Alkali Metals (Group 1): The Kings of Reactivity
The alkali metals, residing in Group 1 of the periodic table (excluding hydrogen), are notorious for their exceptional reactivity. This high reactivity stems from their electronic configuration: they possess only one valence electron, loosely held in their outermost shell. This lone electron is readily lost, forming a +1 ion and resulting in a highly exothermic reaction.
Lithium (Li):
While the least reactive of the alkali metals, lithium is still significantly reactive, especially when exposed to water. The reaction with water is vigorous, producing hydrogen gas and lithium hydroxide. Lithium's relatively low reactivity compared to other alkali metals is due to the strong attraction between its nucleus and its single valence electron. It finds applications in batteries, due to its high electrochemical potential and lightweight nature.
Sodium (Na):
Sodium reacts violently with water, generating substantial heat and producing hydrogen gas and sodium hydroxide. This reaction is often demonstrated in chemistry classrooms, albeit with caution, as it can be quite dramatic. Sodium's reactivity makes it unsuitable for exposure to air or moisture, necessitating storage in inert environments. It plays a vital role in numerous industrial applications, such as the production of sodium hydroxide (lye), which is extensively used in various industrial processes.
Potassium (K):
Potassium exhibits even more vigorous reactivity than sodium. Its reaction with water is extremely rapid and exothermic, often igniting the liberated hydrogen gas. Like sodium, it must be stored under inert conditions to prevent unwanted reactions. Potassium is essential for biological systems, playing a crucial role in nerve impulse transmission and muscle contraction. Despite its reactivity, potassium compounds are widely used in fertilizers.
Rubidium (Rb) and Cesium (Cs):
Rubidium and cesium, situated further down Group 1, display the highest reactivity among the alkali metals. Their reactions with water are explosive, instantly igniting the hydrogen gas produced. Their extremely low ionization energies and large atomic radii contribute to this heightened reactivity. Both rubidium and cesium are rarely encountered in their elemental form due to their extreme instability in air and moisture. Cesium is used in atomic clocks due to its precise atomic transitions.
The Alkaline Earth Metals (Group 2): A Step Down, But Still Reactive
The alkaline earth metals, located in Group 2, also exhibit notable reactivity, although less than the alkali metals. This is because they have two valence electrons, requiring more energy to remove them compared to the single electron in alkali metals. However, their reactivity is still substantial and requires careful handling.
Magnesium (Mg):
Magnesium reacts readily with acids and oxygen, forming magnesium oxide (MgO) upon exposure to air. While not as violent as alkali metal reactions with water, the reaction of magnesium with water is still noticeable, producing magnesium hydroxide and hydrogen gas. Magnesium is a vital component of chlorophyll, essential for photosynthesis in plants. It also finds extensive use in lightweight alloys for aerospace and automotive applications.
Calcium (Ca):
Calcium reacts more vigorously with water than magnesium, although still not as dramatically as the alkali metals. It reacts readily with oxygen and acids, forming calcium oxide and calcium salts, respectively. Calcium is essential for many biological processes, particularly bone formation and muscle contraction. It is also used extensively in construction materials like cement and plaster.
Strontium (Sr) and Barium (Ba):
Strontium and barium exhibit greater reactivity than magnesium and calcium, reacting more vigorously with water and air. Their reactions are noticeably more exothermic and produce greater quantities of hydrogen gas when reacted with water. Strontium compounds are sometimes used in fireworks for their bright red color, while barium sulfate is used as a contrast agent in medical imaging.
The Halogens (Group 17): Highly Reactive Nonmetals
The halogens, located in Group 17, represent another group of highly reactive elements. Unlike the alkali and alkaline earth metals, halogens are nonmetals, achieving stability by gaining an electron to form a -1 ion. Their high electronegativity makes them readily accept electrons from other atoms, leading to vigorous reactions.
Fluorine (F):
Fluorine is the most reactive nonmetal and one of the most reactive elements overall. Its extremely high electronegativity and small atomic radius contribute to its incredible reactivity. It reacts violently with almost all other elements, including noble gases, forming highly stable fluorides. It is exceptionally dangerous and requires specialized handling procedures. Despite its toxicity, fluorine compounds have important applications in various fields, including dentistry (fluoride in toothpaste).
Chlorine (Cl):
Chlorine is less reactive than fluorine but still highly reactive. It readily reacts with many metals and nonmetals, forming chlorides. Chlorine is a well-known disinfectant, used in water treatment and bleaching agents. Its reactivity plays a crucial role in its disinfecting properties.
Bromine (Br) and Iodine (I):
Bromine and iodine are less reactive than chlorine and fluorine, reflecting their lower electronegativity and larger atomic radii. Bromine is a reddish-brown liquid at room temperature, while iodine is a dark gray solid. Both are highly reactive and form stable bromides and iodides. Iodine plays a vital role in human metabolism, and its compounds are used in various applications, including medicine and photography.
Factors Affecting Reactivity
Several factors influence the reactivity of elements:
- Atomic Radius: Larger atoms have their outermost electrons further from the nucleus, leading to weaker attraction and increased reactivity (for metals).
- Electronegativity: Highly electronegative elements readily attract electrons, increasing their reactivity (for nonmetals).
- Ionization Energy: Lower ionization energies indicate that electrons are more easily removed, contributing to higher reactivity (for metals).
- Electron Affinity: High electron affinity signifies a strong tendency to accept electrons, thus increasing reactivity (for nonmetals).
Conclusion
The most reactive elements on the periodic table are those with extreme tendencies to either lose or gain electrons to achieve a stable electron configuration. Alkali metals, with their readily available single valence electron, readily lose electrons, while halogens, with their need for one electron to complete their octet, eagerly accept them. Understanding their reactivity is crucial for predicting their behavior, developing safe handling procedures, and harnessing their unique properties in diverse applications, ranging from everyday items to advanced technologies. While their reactivity can be dangerous, it is also the source of their extraordinary utility and makes them fascinating subjects of scientific study. The periodic table, therefore, serves not only as a catalogue of elements, but as a roadmap to their inherent potential and reactivity, guiding our understanding and manipulation of the material world.
Latest Posts
Latest Posts
-
Speed Of Light In Terms Of Permittivity And Permeability
Mar 17, 2025
-
What Does T Stand For In Physics
Mar 17, 2025
-
How Many Electron Does Oxygen Have
Mar 17, 2025
-
What Is The Difference Between Percentage And Percentile
Mar 17, 2025
-
What Is The Lcm Of 3 5 And 4
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about What Are The Most Reactive Elements On The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
