How Many Electron Does Oxygen Have
Juapaving
Mar 17, 2025 · 6 min read
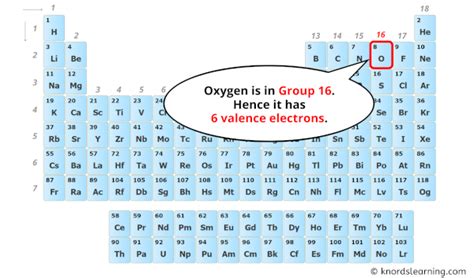
Table of Contents
How Many Electrons Does Oxygen Have? A Deep Dive into Atomic Structure
Oxygen, the life-giving element, plays a crucial role in our existence. But beyond its biological significance, understanding its atomic structure, particularly the number of electrons it possesses, unlocks a deeper appreciation for its chemical behavior and properties. This article delves into the fascinating world of oxygen's electron configuration, exploring its implications for bonding, reactivity, and overall importance in chemistry and beyond.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
Before we pinpoint the number of electrons in oxygen, let's establish a foundational understanding of atomic structure. Every atom is composed of three subatomic particles:
- Protons: Positively charged particles located in the atom's nucleus. The number of protons defines the element's atomic number and its identity.
- Neutrons: Neutral particles (no charge) also residing in the nucleus. They contribute to the atom's mass but not its charge.
- Electrons: Negatively charged particles orbiting the nucleus in specific energy levels or shells. These electrons are responsible for chemical bonding and the element's chemical properties.
The number of protons and electrons in a neutral atom are always equal, ensuring a balanced electrical charge. This balance is crucial for atomic stability.
Oxygen's Atomic Number: The Key to its Electron Count
Oxygen's atomic number is 8. This means a neutral oxygen atom contains eight protons in its nucleus. Because the number of protons equals the number of electrons in a neutral atom, a neutral oxygen atom possesses eight electrons. This fundamental fact is the cornerstone of understanding oxygen's chemical behavior.
Electron Configuration: Where Do Oxygen's Electrons Reside?
While knowing that oxygen has eight electrons is important, understanding their arrangement within the atom is even more crucial. This arrangement, known as the electron configuration, dictates how oxygen interacts with other atoms. The electrons occupy specific energy levels or shells around the nucleus.
Oxygen's electron configuration is 1s²2s²2p⁴. Let's break this down:
- 1s²: The first energy level (n=1) contains a single sublevel, designated as 's'. This sublevel can hold a maximum of two electrons. Oxygen has two electrons in this 1s orbital.
- 2s²: The second energy level (n=2) also contains an 's' sublevel, which can also hold up to two electrons. Oxygen has two electrons in this 2s orbital.
- 2p⁴: The second energy level also contains three 'p' sublevels (2px, 2py, 2pz), each capable of holding up to two electrons. In total, the 'p' sublevel can hold six electrons. Oxygen has four electrons in its 2p orbitals.
This configuration explains oxygen's high reactivity. The 2p sublevel is only half-filled, meaning it has room for two more electrons. This makes oxygen highly likely to form chemical bonds to achieve a stable electron configuration, usually by gaining two electrons to achieve a full octet (eight electrons in its outermost shell).
Oxygen's Reactivity and Chemical Bonding: Implications of its Electron Configuration
Oxygen's incomplete outermost electron shell drives its significant reactivity. To achieve stability, oxygen readily forms chemical bonds with other atoms. The most common ways it achieves this are:
- Ionic Bonding: Oxygen can gain two electrons from another atom, forming a negatively charged ion (anion) with a -2 charge (O²⁻). This is often observed in reactions with alkali metals and alkaline earth metals.
- Covalent Bonding: Oxygen can share electrons with other atoms to complete its octet. This is prevalent in the formation of molecules like water (H₂O) and oxygen gas (O₂). In O₂, two oxygen atoms share two pairs of electrons to achieve a stable configuration.
The electronegativity of oxygen (its tendency to attract electrons in a bond) is relatively high. This contributes to its strong affinity for electrons and its role as an oxidizing agent in many chemical reactions.
Isotopes of Oxygen: Variations in Neutron Count, Not Electron Count
While the number of electrons in a neutral oxygen atom always remains eight, isotopes of oxygen exist. Isotopes are atoms of the same element with the same number of protons but different numbers of neutrons. This means they have different mass numbers but the same chemical properties (largely determined by electron configuration). Common isotopes of oxygen include:
- ¹⁶O: The most abundant isotope, with 8 protons and 8 neutrons.
- ¹⁷O: A less abundant isotope, with 8 protons and 9 neutrons.
- ¹⁸O: Another less abundant isotope, with 8 protons and 10 neutrons.
Despite these variations in neutron count, the number of electrons remains consistent at eight in neutral atoms of all oxygen isotopes. The difference in neutron number affects the atom's mass but not its chemical behavior.
Oxygen's Significance: A Life-Sustaining Element
The eight electrons of oxygen are fundamentally linked to its vital role in supporting life on Earth. Oxygen's high reactivity and its ability to form strong covalent bonds enable it to participate in a vast array of biological processes. Key examples include:
- Cellular Respiration: Oxygen serves as the final electron acceptor in the electron transport chain, a crucial process generating ATP (adenosine triphosphate), the cell's primary energy source.
- Water Formation: Oxygen's participation in water (H₂O) formation is essential for countless biological functions. Water acts as a solvent, reactant, and transport medium within organisms.
- Ozone Layer Formation: Oxygen molecules in the stratosphere form ozone (O₃), a vital layer that absorbs harmful ultraviolet (UV) radiation from the sun.
Beyond the Basics: Advanced Concepts Related to Oxygen's Electrons
Diving deeper, the behavior of oxygen's electrons is explored in more advanced chemistry concepts, including:
- Molecular Orbital Theory: This theory describes the bonding in oxygen molecules (O₂) more accurately than simple Lewis structures. It explains the paramagnetism of oxygen, a property stemming from the presence of unpaired electrons in its molecular orbitals.
- Oxidation States: Oxygen's common oxidation state is -2, reflecting its tendency to gain two electrons in chemical reactions. However, it can exhibit other oxidation states in certain compounds, such as peroxides (-1) and superoxides (-½).
- Spectroscopy: Techniques like photoelectron spectroscopy provide detailed information about the energy levels and binding energies of oxygen's electrons, furthering our understanding of its electronic structure.
Conclusion: The Importance of Understanding Oxygen's Eight Electrons
The seemingly simple fact that oxygen possesses eight electrons has far-reaching consequences. Its electron configuration dictates its reactivity, its ability to form bonds, and ultimately, its indispensable role in sustaining life as we know it. From basic chemical reactions to complex biological processes, understanding the arrangement and behavior of oxygen's electrons is fundamental to comprehending the world around us. Further exploration of these concepts reveals the rich complexity of this essential element and its contribution to a multitude of phenomena in chemistry and biology. By grasping the significance of oxygen's eight electrons, we unlock a deeper appreciation for the elegance and interconnectedness of nature.
Latest Posts
Latest Posts
-
Which Is The Most Fundamental Of The Physical Sciences
Mar 17, 2025
-
Perimeter And Area Of A Triangle
Mar 17, 2025
-
Balanced Equation For Sodium Carbonate And Hydrochloric Acid
Mar 17, 2025
-
A Animal That Lays Eggs And Is Not A Bird
Mar 17, 2025
-
What Part Of The Cell Stores Water
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about How Many Electron Does Oxygen Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
