Unit Of Measurement For Specific Heat Capacity
Juapaving
Mar 26, 2025 · 6 min read
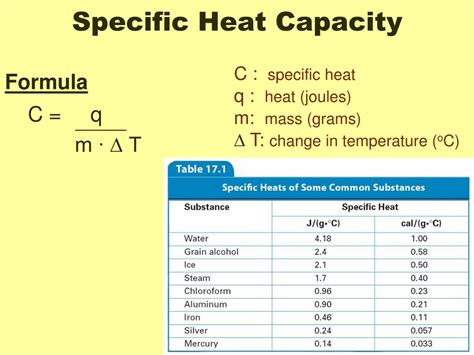
Table of Contents
Unit of Measurement for Specific Heat Capacity: A Comprehensive Guide
Specific heat capacity is a fundamental concept in thermodynamics, representing the amount of heat required to raise the temperature of one unit of mass of a substance by one degree Celsius (or one Kelvin). Understanding its measurement is crucial in various fields, from engineering and materials science to meteorology and climate modeling. This comprehensive guide delves into the units used to express specific heat capacity, exploring their origins, interconversions, and applications.
Understanding Specific Heat Capacity
Before diving into the units, let's solidify our understanding of specific heat capacity itself. It's a material property, meaning it varies depending on the substance. Water, for example, has a relatively high specific heat capacity, meaning it requires a significant amount of heat to change its temperature. This is why water is often used as a coolant. Conversely, metals generally have lower specific heat capacities.
The specific heat capacity is defined by the equation:
c = Q / (mΔT)
Where:
- c represents the specific heat capacity
- Q represents the heat energy transferred (usually measured in Joules)
- m represents the mass of the substance (usually measured in kilograms or grams)
- ΔT represents the change in temperature (measured in degrees Celsius or Kelvin)
Common Units of Specific Heat Capacity
Several units are used to express specific heat capacity, each stemming from different choices for the units of heat energy, mass, and temperature. The most common ones include:
1. Joules per kilogram-kelvin (J/kg·K) or Joules per kilogram-degree Celsius (J/kg·°C)
This is the SI unit (International System of Units) for specific heat capacity. It's widely used in scientific and engineering contexts. The use of Kelvin or Celsius is interchangeable here because the magnitude of a degree Kelvin and a degree Celsius is identical. It represents the amount of heat energy (in Joules) needed to raise the temperature of one kilogram of a substance by one Kelvin (or one degree Celsius).
Example: If a material has a specific heat capacity of 450 J/kg·K, it means that 450 Joules of heat are required to increase the temperature of 1 kilogram of that material by 1 Kelvin.
2. Joules per gram-kelvin (J/g·K) or Joules per gram-degree Celsius (J/g·°C)
This unit is often preferred when dealing with smaller samples or when the mass is more conveniently expressed in grams. The conversion between J/kg·K and J/g·K is straightforward: 1 J/kg·K = 0.001 J/g·K. This unit offers a more practical scale for many experimental scenarios.
Example: A specific heat capacity of 0.45 J/g·K indicates that 0.45 Joules of heat are needed to raise the temperature of 1 gram of the substance by 1 degree Celsius.
3. Calories per gram-degree Celsius (cal/g·°C) or Calories per gram-Kelvin (cal/g·K)
This unit uses the calorie as the unit of heat energy. One calorie (cal) is defined as the amount of heat required to raise the temperature of 1 gram of water by 1 degree Celsius. Historically, the calorie was widely used, particularly in chemistry and biology. However, the SI unit (Joules) is now the preferred unit for scientific work. Note the distinction between the "calorie" used in nutrition (kilocalorie or kcal) and the calorie used in this context.
Example: A specific heat capacity of 1 cal/g·°C indicates that 1 calorie of heat raises the temperature of 1 gram of the substance by 1 degree Celsius.
4. British Thermal Units per pound-degree Fahrenheit (BTU/lb·°F)
This unit is common in the United States and some other countries that use the imperial system of units. A BTU (British Thermal Unit) is the amount of heat required to raise the temperature of one pound of water by one degree Fahrenheit. This unit is used in applications like HVAC (Heating, Ventilation, and Air Conditioning) systems and energy calculations in building design.
Example: A value of 0.24 BTU/lb·°F suggests that 0.24 BTUs of heat are needed to increase the temperature of 1 pound of material by 1 degree Fahrenheit.
Unit Conversions: A Practical Guide
Being able to seamlessly convert between different units of specific heat capacity is essential. Here's a guide to the most common conversions:
1. J/kg·K to J/g·K:
To convert from J/kg·K to J/g·K, simply multiply by 0.001 (or divide by 1000).
Example: 500 J/kg·K = 500 * 0.001 J/g·K = 0.5 J/g·K
2. J/kg·K to cal/g·°C:
To convert from J/kg·K to cal/g·°C, use the conversion factor 1 cal ≈ 4.184 J. Remember that the conversion is needed only for the Joules part.
Example: 450 J/kg·K = (450/1000) J/g·K = (450/1000) / 4.184 cal/g·°C ≈ 0.107 cal/g·°C
3. J/kg·K to BTU/lb·°F:
This conversion is more complex, involving multiple conversion factors. 1 BTU ≈ 1055 J, 1 kg ≈ 2.205 lb, and 1 °C ≈ 1.8 °F. The calculation requires careful attention to the units. Online converters can be incredibly helpful in this case.
4. cal/g·°C to J/g·K:
To convert from cal/g·°C to J/g·K, multiply by 4.184.
Example: 0.5 cal/g·°C = 0.5 * 4.184 J/g·K = 2.092 J/g·K
Importance of Choosing the Right Unit
The choice of unit for specific heat capacity depends heavily on the context. In scientific publications, the SI unit (J/kg·K) is strongly preferred for its clarity and international standardization. However, in certain applications, other units might be more practical or commonly used. Using the appropriate unit ensures clear communication and avoids confusion.
Example: In a materials science experiment involving small samples, using J/g·K might be simpler and more intuitive. In contrast, HVAC calculations might favor BTU/lb·°F due to its prevalent use in the industry.
Applications of Specific Heat Capacity
Understanding specific heat capacity has vast applications across numerous scientific and engineering disciplines:
- Material Science: Determining the specific heat capacity of materials is essential for designing materials with specific thermal properties. This knowledge is critical in applications ranging from heat sinks to thermal insulation.
- Chemical Engineering: Specific heat capacity is crucial in process design, reaction kinetics, and heat exchanger calculations in chemical processing plants.
- Climate Modeling: The specific heat capacity of water plays a significant role in climate models, influencing the Earth's temperature regulation.
- Meteorology: Understanding the specific heat capacity of air is critical in weather forecasting and climate studies.
- Mechanical Engineering: Specific heat capacity is used extensively in engine design, heat transfer analysis, and thermodynamic efficiency calculations.
- Food Science: Knowing the specific heat capacity of foods is important in food processing, preservation, and cooking.
- Medical Applications: Specific heat capacity considerations are vital in designing medical devices, understanding thermal interactions with tissues, and developing cryogenic therapies.
The choice of appropriate unit for specific heat capacity is dictated by the specific application. Using the wrong unit can lead to errors in calculations and potentially flawed conclusions. Therefore, attention to units and their conversions is paramount for accuracy and reliability in any field utilizing this fundamental property.
Conclusion
Specific heat capacity is a vital concept with far-reaching applications. Mastering the various units used to express it, understanding their interconversions, and knowing when to apply each unit is crucial for success in many scientific and engineering disciplines. While J/kg·K is the preferred SI unit, the context of the problem dictates the most appropriate unit for clear communication and accurate calculations. Consistent attention to detail regarding units ensures reliable results and fosters a deeper understanding of this critical thermophysical property.
Latest Posts
Latest Posts
-
The Distance Around A Figure Is Called
Mar 29, 2025
-
Is Blood A Compound Or Mixture
Mar 29, 2025
-
What Can 17 Be Divided By
Mar 29, 2025
-
How Many Hearts Does Wormsn Have
Mar 29, 2025
-
Write The Prime Factorization Of 75
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Unit Of Measurement For Specific Heat Capacity . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
