Is Blood A Compound Or Mixture
Juapaving
Mar 29, 2025 · 5 min read
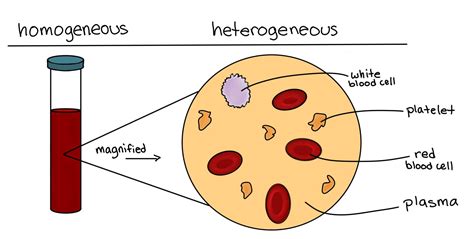
Table of Contents
Is Blood a Compound or a Mixture? Unraveling the Composition of Life's River
Blood, the vibrant crimson fluid coursing through our veins, is far more complex than a simple compound or mixture. While it might seem straightforward to categorize it as one or the other, the reality is far more nuanced. This article delves deep into the composition of blood, examining its various components to understand why it defies simple classification and why it's crucial to appreciate its intricate nature for both scientific understanding and medical applications.
The Fundamental Difference: Compounds vs. Mixtures
Before we dive into the intricacies of blood, let's establish the fundamental distinction between compounds and mixtures.
-
Compounds: Compounds are formed when two or more elements chemically combine in fixed proportions, resulting in a new substance with properties distinct from its constituent elements. Think of water (H₂O): two hydrogen atoms and one oxygen atom bond chemically to create a substance with entirely different properties than hydrogen gas or oxygen gas. The composition of a compound is always consistent.
-
Mixtures: Mixtures are formed when two or more substances are physically combined, retaining their individual properties. A salad, for example, is a mixture of various vegetables—each retaining its unique characteristics. Unlike compounds, mixtures can have varying compositions.
Deconstructing Blood: A Closer Look at its Components
Blood is a complex fluid connective tissue, transporting essential substances throughout the body. To understand its classification, we need to examine its key components:
1. Plasma: The Liquid Matrix
Plasma constitutes about 55% of blood volume. It's a straw-colored liquid primarily composed of water (about 92%), but also contains a significant amount of dissolved substances:
-
Proteins: Albumin, globulins, and fibrinogen are vital proteins in plasma. Albumin maintains osmotic pressure, globulins play a role in immune function and transport, and fibrinogen is crucial for blood clotting. The presence of numerous proteins with differing functions already complicates the simple compound/mixture dichotomy.
-
Electrolytes: Ions like sodium, potassium, chloride, calcium, and bicarbonate maintain fluid balance, nerve impulse transmission, and muscle contraction. These are essential for countless bodily functions.
-
Nutrients: Glucose, amino acids, lipids, and vitamins are carried by plasma to nourish cells throughout the body. The variable concentrations of these nutrients based on dietary intake further support a mixture classification.
-
Waste Products: Urea, creatinine, and uric acid are transported to the kidneys for excretion. Again, the variable levels of these waste products reinforce the idea of blood as a mixture.
-
Gases: Oxygen and carbon dioxide are crucial for cellular respiration. Their concentrations fluctuate depending on metabolic activity and respiratory function.
2. Formed Elements: The Cellular Components
The remaining 45% of blood consists of formed elements:
-
Red Blood Cells (Erythrocytes): These are the most abundant cells, responsible for oxygen transport. They contain hemoglobin, a protein that binds to oxygen. The consistent chemical structure of hemoglobin might suggest a compound-like aspect, but their presence within a variable fluid matrix pushes towards mixture classification.
-
White Blood Cells (Leukocytes): These are part of the immune system, defending against infection. There are various types of leukocytes, each with specific functions. The diverse range of white blood cell types further supports the mixture classification.
-
Platelets (Thrombocytes): These small cell fragments are essential for blood clotting. Their involvement in a complex cascade of reactions, rather than a single chemical bond, reinforces the mixture nature of blood.
Why Blood is Primarily Considered a Mixture
Based on the above analysis, it's clear that blood is predominantly a mixture. Several compelling reasons support this classification:
-
Variable Composition: The concentrations of various components in plasma, such as nutrients, waste products, and gases, fluctuate continuously depending on factors like diet, metabolic activity, and respiration. This inherent variability is characteristic of mixtures, not compounds.
-
Physical Combination: The formed elements (red blood cells, white blood cells, and platelets) are suspended in plasma—a physical combination, not a chemical bond. They maintain their individual identities and functions within the blood.
-
Retention of Individual Properties: Each component of blood retains its unique properties. The proteins in plasma function differently from the oxygen-carrying capacity of red blood cells. This independent functioning is indicative of a mixture.
The Nuances: A Complex Mixture, Not a Simple One
While the overall classification is a mixture, it's important to acknowledge the complexity. Blood isn't a simple, homogenous mixture like saltwater. It's a highly heterogeneous mixture containing a diverse array of components that interact in intricate ways. The presence of proteins with specific chemical structures and the chemical reactions within the clotting cascade introduce elements of complexity. However, the overall variability in composition and the physical suspension of cells within a fluid matrix strongly favor the mixture classification.
The Importance of Understanding Blood's Composition
The understanding that blood is primarily a mixture is crucial in various fields:
-
Medical Diagnosis: Blood tests analyze various components to diagnose different health conditions. The variability in composition, a key characteristic of a mixture, is essential in identifying abnormalities.
-
Blood Transfusions: Successful blood transfusions require careful matching of blood types due to the presence of different antigens on red blood cells. This complex mixture's nature mandates careful compatibility analysis.
-
Research and Development: Understanding blood's composition is essential for ongoing research in hematology, immunology, and other related fields. The complex interactions within the blood mixture are crucial for drug development and therapeutic advancements.
-
Forensic Science: Blood analysis plays a vital role in forensic investigations, leveraging the unique composition of blood to provide crucial evidence. The analysis of various components aids in determining aspects such as blood type, the presence of alcohol or drugs, and even the time since the event occurred.
Conclusion: A Complex and Dynamic Mixture
In conclusion, blood is best classified as a heterogeneous mixture. While its components exhibit complex interactions and some elements possess specific chemical structures, the variability in composition, the physical combination of its components, and the retention of individual properties strongly support this classification. Recognizing the nuanced nature of blood as a complex mixture is fundamental to understanding its physiological significance and crucial for various scientific and medical applications. Further research into the intricate interplay within this complex mixture continues to unveil its secrets and offers potential for advancements in numerous health-related areas.
Latest Posts
Latest Posts
-
How Many Hours Is 1800 Minutes
Mar 31, 2025
-
In Glycolysis There Is A Net Gain Of Atp
Mar 31, 2025
-
The Clavicle Articulates With The Sternum And The Scapula
Mar 31, 2025
-
Is Oxygen A Metal Nonmetal Or Metalloid
Mar 31, 2025
-
Keyboard Is A Hardware Or Software
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Is Blood A Compound Or Mixture . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
