Types Of Chemical Reactions Worksheet Answers
Juapaving
Mar 20, 2025 · 7 min read
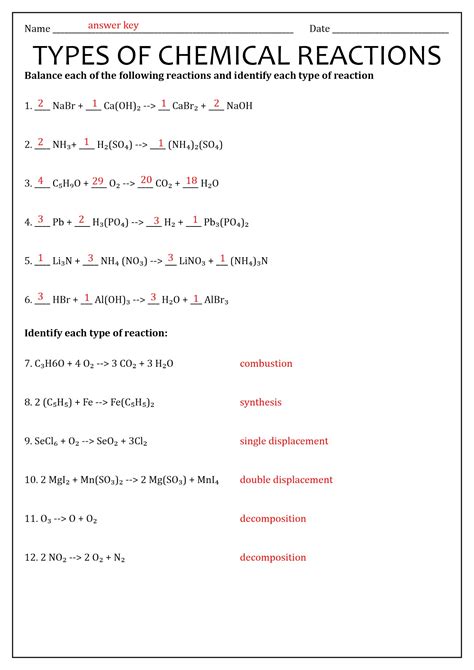
Table of Contents
Types of Chemical Reactions Worksheet Answers: A Comprehensive Guide
Understanding chemical reactions is fundamental to grasping chemistry. This guide provides comprehensive answers and explanations for a typical "Types of Chemical Reactions" worksheet, covering various reaction types with examples. We'll delve into the nuances of each reaction, ensuring a solid understanding of the underlying principles. This detailed explanation will serve as a valuable resource for students and anyone looking to solidify their knowledge of chemical reactions.
Key Concepts Before We Begin
Before diving into specific reaction types, let's refresh some key concepts:
- Reactants: These are the substances that undergo change during a chemical reaction. They're found on the left side of the chemical equation.
- Products: These are the new substances formed as a result of the chemical reaction. They're on the right side of the equation.
- Chemical Equation: A symbolic representation of a chemical reaction, showing reactants and products with their stoichiometric coefficients (the numbers in front of the chemical formulas). A balanced equation ensures the law of conservation of mass is obeyed (same number and type of atoms on both sides).
Types of Chemical Reactions and Worksheet Answers
Now, let's tackle the different types of chemical reactions typically found on worksheets:
1. Synthesis (Combination) Reactions
Definition: Two or more reactants combine to form a single product.
General Form: A + B → AB
Examples & Explanations:
- Formation of Water: 2H₂ + O₂ → 2H₂O Two molecules of hydrogen gas react with one molecule of oxygen gas to produce two molecules of water. This is a highly exothermic reaction, releasing significant energy.
- Formation of Magnesium Oxide: 2Mg + O₂ → 2MgO Magnesium metal reacts with oxygen to form magnesium oxide. This is a classic example of a synthesis reaction involving a metal and a nonmetal.
- Formation of Sodium Chloride: 2Na + Cl₂ → 2NaCl Sodium metal reacts vigorously with chlorine gas to form sodium chloride (table salt). This reaction is also exothermic and demonstrates the formation of an ionic compound.
Worksheet Answer Guidance: Identify reactions where multiple reactants combine to produce a single, more complex product. Look for patterns where elements combine directly or simple compounds combine to create a larger molecule.
2. Decomposition Reactions
Definition: A single reactant breaks down into two or more simpler products.
General Form: AB → A + B
Examples & Explanations:
- Electrolysis of Water: 2H₂O → 2H₂ + O₂ The application of an electric current breaks down water into its constituent elements, hydrogen and oxygen. This demonstrates the use of energy to drive a decomposition reaction.
- Decomposition of Calcium Carbonate: CaCO₃ → CaO + CO₂ Heating calcium carbonate (limestone) leads to its decomposition into calcium oxide (quicklime) and carbon dioxide. This reaction is often used in industrial processes.
- Decomposition of Hydrogen Peroxide: 2H₂O₂ → 2H₂O + O₂ Hydrogen peroxide decomposes slowly into water and oxygen gas, often catalyzed by enzymes or transition metal ions.
Worksheet Answer Guidance: Look for reactions where a single compound breaks down into two or more simpler substances. The application of heat or electricity often indicates a decomposition reaction.
3. Single Displacement (Replacement) Reactions
Definition: One element replaces another element in a compound.
General Form: A + BC → AC + B
Examples & Explanations:
- Reaction of Zinc with Hydrochloric Acid: Zn + 2HCl → ZnCl₂ + H₂ Zinc metal reacts with hydrochloric acid, replacing hydrogen to form zinc chloride and hydrogen gas. This is a common example used to illustrate the reactivity series of metals.
- Reaction of Iron with Copper(II) Sulfate: Fe + CuSO₄ → FeSO₄ + Cu Iron reacts with copper(II) sulfate solution to displace copper, forming iron(II) sulfate and copper metal. This reaction demonstrates the relative reactivity of iron and copper.
- Reaction of Chlorine with Sodium Bromide: Cl₂ + 2NaBr → 2NaCl + Br₂ Chlorine reacts with sodium bromide, displacing bromine to form sodium chloride and bromine gas. This showcases the higher reactivity of chlorine compared to bromine.
Worksheet Answer Guidance: Identify reactions where one element replaces another in a compound. The reactivity series of metals and halogens are crucial in predicting the outcome of such reactions.
4. Double Displacement (Metathesis) Reactions
Definition: Two compounds exchange ions to form two new compounds.
General Form: AB + CD → AD + CB
Examples & Explanations:
- Reaction of Silver Nitrate with Sodium Chloride: AgNO₃ + NaCl → AgCl + NaNO₃ Silver nitrate reacts with sodium chloride to form silver chloride (a precipitate) and sodium nitrate. This is a classic example used to demonstrate precipitation reactions.
- Reaction of Hydrochloric Acid with Sodium Hydroxide: HCl + NaOH → NaCl + H₂O Hydrochloric acid reacts with sodium hydroxide to form sodium chloride and water. This is a neutralization reaction, a specific type of double displacement reaction.
- Reaction of Barium Chloride with Sodium Sulfate: BaCl₂ + Na₂SO₄ → BaSO₄ + 2NaCl Barium chloride reacts with sodium sulfate to form barium sulfate (another precipitate) and sodium chloride. This reaction is also driven by the formation of an insoluble product.
Worksheet Answer Guidance: Look for reactions where cations and anions switch places between two reactants, resulting in the formation of two new compounds. Precipitate formation, gas evolution, or water formation are often indicators of double displacement reactions.
5. Combustion Reactions
Definition: A rapid reaction with oxygen that produces heat and light. Often involves organic compounds.
General Form: Fuel + O₂ → CO₂ + H₂O + Heat + Light
Examples & Explanations:
- Combustion of Methane: CH₄ + 2O₂ → CO₂ + 2H₂O Methane (natural gas) burns in oxygen to produce carbon dioxide, water, heat, and light. This is a highly exothermic reaction crucial for energy production.
- Combustion of Propane: C₃H₈ + 5O₂ → 3CO₂ + 4H₂O Propane (used in gas grills and heating) combusts in oxygen to produce carbon dioxide, water, heat, and light. This reaction also releases significant energy.
- Combustion of Ethanol: C₂H₅OH + 3O₂ → 2CO₂ + 3H₂O Ethanol (alcohol) burns in oxygen producing carbon dioxide, water, heat, and light. This reaction is similar to the combustion of other hydrocarbons.
Worksheet Answer Guidance: Identify reactions involving a rapid reaction with oxygen, usually producing carbon dioxide and water as products, along with heat and light. These reactions typically involve organic molecules as the fuel source.
6. Acid-Base Reactions (Neutralization)
Definition: A reaction between an acid and a base, usually producing water and a salt. This is a specific type of double displacement reaction.
General Form: Acid + Base → Salt + Water
Examples & Explanations:
- Reaction of Hydrochloric Acid with Sodium Hydroxide: HCl(aq) + NaOH(aq) → NaCl(aq) + H₂O(l) Hydrochloric acid reacts with sodium hydroxide to form sodium chloride and water. The (aq) denotes aqueous solution, and (l) denotes liquid.
- Reaction of Sulfuric Acid with Potassium Hydroxide: H₂SO₄(aq) + 2KOH(aq) → K₂SO₄(aq) + 2H₂O(l) Sulfuric acid reacts with potassium hydroxide to produce potassium sulfate and water. Note the stoichiometry – two moles of KOH are required to neutralize one mole of H₂SO₄.
- Reaction of Acetic Acid with Sodium Hydroxide: CH₃COOH(aq) + NaOH(aq) → CH₃COONa(aq) + H₂O(l) Acetic acid (vinegar) reacts with sodium hydroxide to produce sodium acetate and water.
Worksheet Answer Guidance: Look for reactions between acids (containing H⁺) and bases (containing OH⁻), producing water and a salt as the products. The pH change from acidic to neutral or basic is also indicative.
Advanced Considerations and Challenges
Some worksheets may include more complex scenarios or reactions that require deeper understanding:
- Redox Reactions (Oxidation-Reduction): These involve the transfer of electrons between species. One species is oxidized (loses electrons), and another is reduced (gains electrons). Many single displacement and combustion reactions are also redox reactions.
- Identifying Limiting Reactants: Determining which reactant will be completely consumed in a reaction, limiting the amount of product formed.
- Stoichiometric Calculations: Using balanced chemical equations to calculate the amounts of reactants and products involved in a reaction.
Conclusion
This comprehensive guide provides detailed answers and explanations for a typical "Types of Chemical Reactions" worksheet. By understanding the characteristics and examples of each reaction type—synthesis, decomposition, single displacement, double displacement, combustion, and acid-base neutralization—you can confidently approach similar problems. Remember to practice identifying the key features of each type of reaction, which will strengthen your understanding of chemical processes. This understanding is crucial for further studies in chemistry and related fields. Remember to always double-check your work and consult your textbook or teacher for further clarification if needed.
Latest Posts
Latest Posts
-
Is Coal The Same As Charcoal
Mar 20, 2025
-
In Which Organelle Does Photosynthesis Take Place
Mar 20, 2025
-
Is Dry Ice An Element Compound Or Mixture
Mar 20, 2025
-
Questions On Balancing Chemical Equations With Answers
Mar 20, 2025
-
Volume Of A Bcc Unit Cell
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about Types Of Chemical Reactions Worksheet Answers . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
